The etonogestrel implant is a subdermal, single-rod contraceptive that releases etonogestrel1. It is considered a very effective and safe contraceptive and moderate or severe side effects rarely occur2-4. We describe a case of allergic reaction to etonogestrel implant that resolved after its extraction.
A a 23-year-old African woman (gravida 0, para 0) presented at our emergency department with severe pain, erythema and difficulty moving the right arm about 72 hours after the etonogestrel-releasing implant had been inserted. The implant had been inserted under local anesthesia with 2% lidocaine in our department. The patient denied history of arm trauma or injury as well as fever or respiratory symptoms. The woman had no relevant medical history, including allergies.
A clinical examination was performed and revealed a skin lesion located on the right arm, around the etonogestrel-releasing implant introduction site. The site, 50 mm long, was characterized by an oval edema with an irregular contour and an erythematous halo associated with intense pain on palpation (Figure 1A). The implant rod was correctly placed and was palpable.
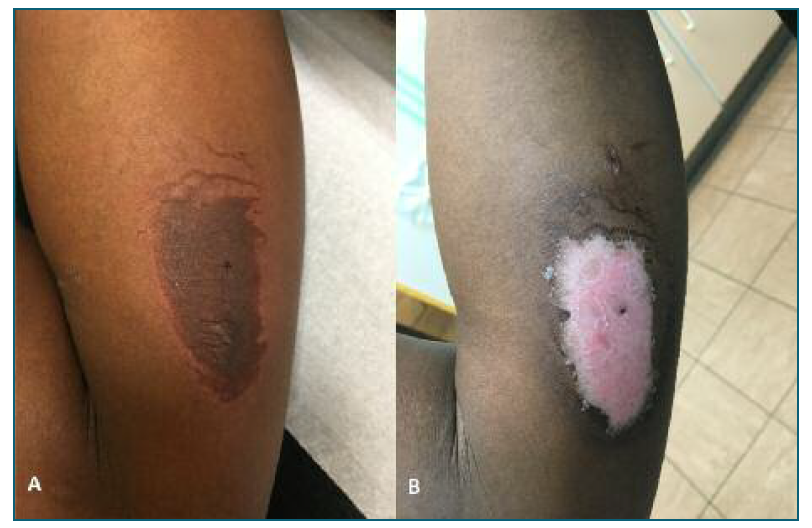
Figure 1 (A) Skin lesion around the etonogestrel-releasing implant introduction site. (B) Follow-up a week later: skin with no signs of inflammation.
The implant was easily removed according with the standard recommendations. Additionally, 1cc of lidocaine was introduced in the contralateral arm to assess a possible hypersensitivity reaction to that drug. None additional medical therapeutic was done.
The patient was reevaluated a week later and reported clear improvement in symptoms. Upon observation, the original skin lesion was now scaly, painless and with no signs of inflammation (Figure 1B). Neither the implant extraction point nor the lidocaine injection point (contralateral arm) presented any changes.
The etonogestrel implant is a subdermal, single-rod contraceptive that releases etonogestrel. Each implant contains 68 mg of the active substance etonogestrel, ethylene vinyl acetate copolymer, barium sulfate and magnesium stearate5. The occurrence of local adverse reactions are described in the literature, however moderate or severe side effects rarely occur1.
We describe a case with local signs and symptoms progressive and severe, completely solved after the extraction of the implant. The clinical presentation strongly correlated with an allergic reaction to one of the components of the etonogestrel implant, which is rare2-4.
The etonogestrel implant is highly regarded for its efficacy and safety, which is reflected in its wide worldwide use. However, the risk of allergic skin reaction should not be overlooked.














