Introduction
Hyperreactio luteinalis (HL) is a rare pregnancy-related entity characterized by a massive enlargement of the ovaries due to the development of numerous benign theca lutein cysts (TLC)1. Although the exact etiology of HL is unknown, TLC are thought to be associated with high levels of circulating human chorionic gonadotrophin (hCG) or increased ovarian sensitivity to hCG. Thereby, conditions in which hCG levels are markedly increased, such as gestational trophoblastic disease and multiple pregnancy, are potential risk factors for HL2,3,4. However, most cases reported in the literature were identified in uncomplicated singleton pregnancies5.
Typically, women with HL remain asymptomatic and the diagnosis is often an incidental finding at the time of third trimester ultrasound or cesarean section1,5. Nausea, vomiting, lower abdominal pain and signs of maternal and fetal virilization may be related to this condition5. Most commonly, both ovaries are affected with multiple ovarian cysts that spontaneously regress in the postpartum period - this is the natural evolution of HL1,5. Ovarian hyperstimulation syndrome (OHSS) is also a self-limiting disorder and is a differential diagnosis to consider6,7. As it is a rare entity, HL ultrasound findings may be erroneously related to an ovarian malignancy, culminating in unnecessary surgical interventions1,8.
In this article, we report a rare case of a woman with bilateral enlargement of the ovaries in a spontaneous singleton pregnancy and the conservative management of this condition.
Case-report
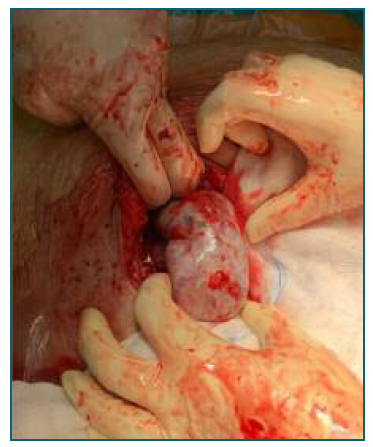
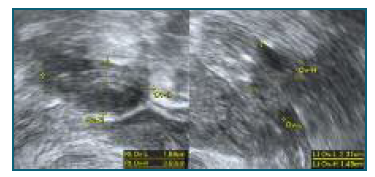
A 35-year-old woman, obese (class II) and asthmatic, gravida 4 para 2 (two term cesarean deliveries), with a spontaneous singleton pregnancy, presented, in the third trimester scan, a bilateral enlargement of the ovaries. No fetal anomalies were found in the first and second trimester scans nor an enlargement of the maternal ovaries was noted before. During the second trimester, the diagnosis of gestational diabetes was made, which was treated with diet and lifestyle changes. At 31 weeks, the patient reported nausea, abdominal tightness, alopecia and hirsutism, more prominent in the abdomen. On physical examination, an abdominal diffuse tenderness with no signs of peritoneal irritation or abdominal masses was observed. On the third trimester scan, the male fetus was adequate in size for the gestational age and no structural abnormalities were detected. Incidentally, it was reported bilateral enlarged maternal ovaries with multiple, apparently simple, cysts (Figure 1). The maximum diameter of the ovaries was about 9 cm and no abnormal vascularity was noted with color Doppler. Physical examination and transabdominal ultrasound assessment were limited by the pregnant uterus and the maternal biotype, therefore a pelvic magnetic resonance imaging (MRI) was performed to better characterize the adnexal masses. MRI confirmed a marked enlargement of the ovaries (the right with 83x46x38 mm and the left with 100x45x36 mm) by multiple thin-walled simple cysts with variable dimensions (Figure 2). No solid components or calcifications in the cysts or ascites were noted. Due to the previous surgical history, the significant increase in abdominal pain and the recurrence of severe nausea and vomiting, causing difficulties in metabolic control, an elective cesarean section was performed at 37 weeks of gestation. A healthy male infant weighing 3175 grams was born. Intraoperatively, voluminous ovaries with multiple simple cysts were found (Figure 3) in the pelvic cavity, with multiple adhesions to the pelvic and uterine walls and colon. The ovaries were left in situ, and the surgery proceeded without intraoperative complications. The patient was discharged home on postoperative day 3. A delay in lactogenesis was observed during hospitalization and, despite professional support, our patient decided not to breastfeed. Her hyperandrogenic and virilization signs spontaneously regressed in a month. The follow-up transvaginal ultrasound was performed 9 weeks postpartum and revealed ovaries with normal morphology and size (the right with 23x19x9 mm and the left with 23x14x11 mm) (Figure 4).
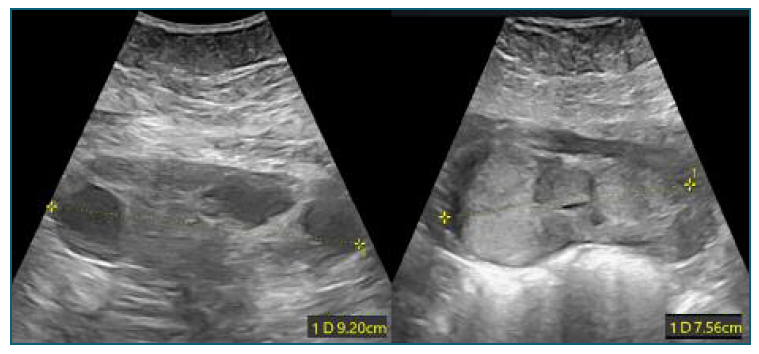
Figure 1 Transabdominal ultrasound at 31 weeks: bilateral enlargement of maternal ovaries with multiple cysts.
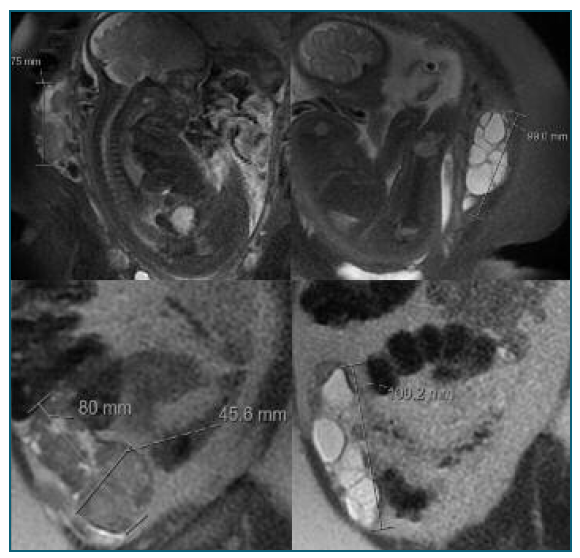
Figure 2 Magnetic resonance imaging confirming the enlargement of the ovaries due to multiple thin-walled cysts.
Discussion
The clinical signs and imaging findings in our patient raised the suspicion of HL, which is a rare, benign and self-limiting syndrome occurring in pregnancy1. High circulating levels of hCG or the ovarian hypersensitivity to this hormone lead to the development of multiple thin-walled TLCs, which may be manifested by bilateral ovarian enlargement during pregnancy1,2. Conditions associated with high levels of serum hCG, such as multiple pregnancy, gestational trophoblastic disease, hydrops fetalis and chronic renal disease or ovarian stroma’s hypersensitivity to hCG, as in polycystic ovarian disease, increase the risk of TLCs formation3,4. Concurrently, ovarian theca cells are a source of androgens, thus clinical features of hyperandrogenism including acne, hirsutism, alopecia, clitoromegaly and voice deepening are expected in women with HL1,9,10. However, those virilization signs are only reported in about 30% of cases since there are several protective mechanisms that decrease serum levels of androgens, including a high circulating concentration of sex hormone-binding globulin (binds to androgens and inactivates its biological activity), progesterone competition for androgen receptors and placental androgen aromatization (transforms the androgens into estrogens)1,5,10. Our patient revealed hirsutism, alopecia and severe nausea and vomiting in the third trimester, which might be related to increased levels of androgens and hCG. Unfortunately, serum hormonal evaluations were not requested to confirm potentially increased values. A hyperandrogenic environment, particularly in the first trimester, may also cause signs of virilization in a female fetus, such as clitoromegaly and labioscrotal fusion5. Since HL usually occurs in the third trimester, these virilization signs are rarely reported. However, in cases of maternal hyperandrogenism, subsequent obstetric scans should screen for signs of fetal virilization that may require postpartum surgical correction1,5. Our patient’s fetus was male, therefore this evaluation was not conducted.
Malinowski et al. reported that HL was associated with fetal growth restriction in 32%, preterm delivery in 38%, and preeclampsia in 19% of pregnancies, hypothesizing a relationship between those pregnancy complications and elevated hCG levels5. Our patient did not present any of these comorbidities, however her pregnancy was complicated by gestational diabetes which has also been reported in other cases of HL5.
Most cases of ovarian enlargement in pregnancy are a consequence of hormonal derangement. However, HL may mimic ovarian malignancy and careful evaluation is essential to avoid unnecessary surgeries. In the past, diagnosis was often made only in cesarean section and surgical intervention was much more frequent11. Nowadays, acknowledgment about HL, especially its clinical presentation, natural history and imaging findings, has allowed its diagnosis to be made earlier. Bilaterally enlarged ovaries composed of thin-walled TLCs (spoke-wheel sign), absence of solid components and physiological flow in Doppler velocimetry are characteristic ultrasound findings of HL12. Laboratory tests, such as hormonal study and tumor markers dosing may complement the assessment. Maternal serum CA-125 concentrations are influenced by pregnancy and may be physiologically increased, especially in the first trimester13. Therefore, CA-125 evaluation may not facilitate the discrimination between an ovarian malignancy and a benign entity. There are reported cases of large adnexal masses in pregnancy with increased CA-125 levels that have undergone cystectomies or oophorectomies, with a histopathologic result compatible with TLCs8,14.
OHSS is a differential diagnosis to consider when pelvic masses are present during pregnancy6. In most cases, OHSS is iatrogenic due to complications of ovulation induction therapy, however it may rarely occur in a spontaneous pregnancy. FSH/LH ovarian receptor mutation is a potential mechanism explaining the genesis of spontaneous OHSS. Unlike HL, OHSS typically presents in the first trimester of pregnancy. Furthermore, HL is characterized by a more indolent clinical course, whereas OHSS may present with acute symptoms, such as ascites, hypovolemia and other important fluid and electrolyte imbalances. Termination of pregnancy is inevitable in some cases of OHSS5,7,15.
In our patient’s ultrasound evaluation, the bilaterality of the findings, the presence of simple, homogeneous and thin-walled cysts and the physiological vascularization pattern, raised the hypothesis of a benign condition. MRI was useful to better characterize the adnexal masses and rule out ovarian malignancy. Despite being a rare disorder, with the current knowledge of the characteristics and evolution of HL, clinicians should be able to make a diagnosis with confidence based on clinical and imaging findings, avoiding unnecessary biopsies or surgical interventions11. Surgery should be reserved for a complication, such as ovarian torsion, pelvic entrapment of the enlarged ovary, cyst rupture or hemorrhage16,17. Therefore, we have decided a conservative and expectant management, considering the benignity and spontaneous postpartum regression. A cesarean section was performed because of our patient’s obstetrical history. In fact, HL does not contraindicate a vaginal delivery, however enlarged ovaries within the abdomen and pelvis may cause obstruction to fetal descent or fetal malpresentation5.
As in our patient, there are reported cases of delayed lactation in HL caused by the high maternal levels of testosterone and hCG. Considering the benefits of maternal breastmilk, women with HL should have professional guidance to encourage breastfeeding5,12.
In conclusion, clinicians must be aware of HL when enlarged ovaries are identified in pregnancy. Since it is an extremely rare entity, it is essential to emphasize that HL is a benign and self-limited condition, with an indication for conservative management. This avoids morbidity associated with unnecessary interventions, such as cystectomies or oophorectomies, which may negatively impact women’s obstetrical and gynecological future.
Authors’ contribution
Luís Ferreira Castro: Contributed to the analysis and interpretation of data, reviewing the literature and drafting the article.
Ana Andrade: Contributed to the critical review of the article
Gonçalo Inocêncio: Contributed to the diagnosis of the clinical case and interpretation of data.
Inês Nunes: Contributed to the diagnosis of the clinical case, critical review and final approval of the submitted version
Jorge Braga: Contributed to the critical review of the article