Introduction
The use of industrial liquid silicone as a body contour modifier is a practice that reached its highest point in the 60s and 70s. After local and systemic complications were reported, entities such as the Food and Drug Administration (FDA), in the United States and Dimed, in Brazil, banned the use of this substance in humans, being the only exception the cases of retinal detachment1.
This report exposes the case of a woman referred to the Senology Unit of the Gynecology Service due to imaging changes resulting from the injection of the referred substance.
Clinical report
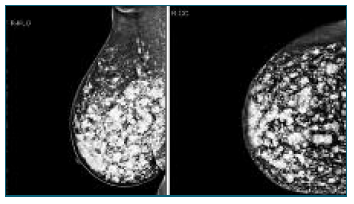
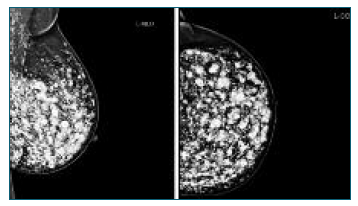
Female patient, 44 years old, with no relevant personal or family history, is conducted to the attending physician to the Senology Service by bilateral BIRADS (Breast Imaging-Reporting and Data System) 3 in breast study (ultrasound and mammography) - Figures 1 and 2.
She had acyclic mastalgia and, on physical examination, palpable painful nodules in both breasts, especially in the lower quadrants, and changes in their contours. The patient mentioned progressive worsening of pain complaints and history of liquid silicone injection in Brazil 19 years before.
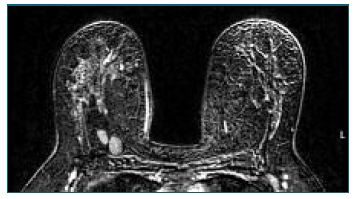
A complementary breast study with magnetic resonance imaging (MRI) was requested, which showed significant bilateral breast dysmorphia due to the presence of countless sites of hyperintense content on T2 in relation to the injection of exogenous material, as well as some areas of greater encapsulation of residual breast tissue - Figure 3.
The case was discussed in a multidisciplinary team and the patient was explained that the conventional breast study with ultrasound and mammography does not allow a safe surveillance of the documented alterations. Thus, in the first line, simple bilateral mastectomy was advised followed by reconstruction with an implant or, alternatively, breast surveillance by MRI. The patient opted for clinical and imaging surveillance, maintaining follow-up in the unit.
Discussion
The use of industrial liquid silicone for aesthetic purposes, despite being illegal, is still practiced in some countries, especially in South America and Asia, attracting some population subgroups because it seems to be an economical alternative compared to the surgical approach. The clinical picture resulting from the procedure may manifest itself in the acute phase or years later, with latency periods of up to 25 years described in the literature2. Complications resulting from inadvertent subcutaneous or intramuscular administration may be local (formation of abscesses, fistulas or granulomas, necrosis, dyschromia, fibrosis with calcification and formation of scarring) or systemic (systemic inflammatory response syndrome, acute/chronic pneumonitis, impairment of lymphatic drainage, embolism and death) 3.
The increase in the incidence of breast cancer because of the injection of industrial silicone remains a controversial topic in the literature. However, regardless of this hypothesis, due to difficulty in diagnostic investigation by conventional imaging methods (ultrasound and mammography) as well as by histopathological examination, an increase in the proportion of false negatives is expected4. Although there is no systematic protocol for the treatment of this condition, owing to the limitations of screening tests and the difficulty or impossibility of removing the injected material, it seems that an early surgical approach with mastectomy is the best procedure in these cases5.
In short, with the current trend of globalization and human mobility, it is essential that health professionals, especially those dedicated to breast pathology, are aware of this practice, of its possible consequences and know how to guide the surveillance and/or therapy of these patients.
Authors’ contributions
Inês Vieira Martins - contributed to the analysis and interpretation of data, reviewing the literature and drafting the article.
Júlio Caldas - contributed to the critical review of the article.
Filomena Nunes - contributed to the critical review of the article.