Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries, with a growing prevalence due to high rates of obesity combined with extended life expectancy1. Its estimated incidence and standardized mortality rate in Portugal are 9.9/100000 and 2.0/100000, respectively, according to Globocan 20202.
Although the majority of cases are detected early, with a low risk of lymph node invasion3, lymph node metastases are an important factor, not only to provide information about staging and prognosis, but also to select adjuvant treatment4. Thus, according to the International Federation of Gynaecology and Obstetrics (FIGO) 2009, the standard staging for endometrial cancer is surgical, through total hysterectomy with bilateral salpingo-oophorectomy and pelvic and para-aortic lymphadenectomy (LDN) 5.
Since nodal metastasis in early-stage EC are rare, systematic lymph node removal changes preoperative staging in less than 10% of cases6. Furthermore, a Co-chrane review from 2017, as well as two recently published studies, revealed that LDN did not improve overall and disease-free survival of patients with presumed stage I disease6-8. On the contrary, it was associated with a higher incidence of serious short and long-term adverse events, such as surgery-related systemic morbidity and lymphoedema formation6,9.
Sentinel lymph node biopsy (SLNB) has therefore been proposed as a less invasive strategy for nodal status assessment. Multiple recent studies demonstrated the advantages of this technique when applied in early-stage endometrial cancer, reporting a high sensitivity, ranging from 91 to 100%, and a negative predictive value of 98-100%10-12. Additionally, the ultrastaging technique allows an increase in the detection of malignant cells in sentinel lymph nodes (SLN) compared to the standard histologic examination of non-sentinel lymph nodes (LN) 13-15.
According to multiple research projects and guidelines, indocyanine green (ICG) dye, injected in the cervix, with detection of the SLN under near-infrared (NIR) light, is the recommended tracer in minimally invasive surgery13,14,16-18, providing the highest detection rate, with a low incidence of adverse effects13,19,20.
Recent international guidelines updated their recommendations regarding lymph node assessment in endometrial cancer: the guideline from the European Society of Gynaecological Oncology (ESGO), European Society for Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP) recommends SLNB for patients with low-risk/intermediate-risk disease, advising against systematic LDN in this group21. For patients with high-intermediate/high-risk disease lymph node biopsy is considered an acceptable alternative to systematic lymphadenectomy for lymph node staging in stage I/II21. The National Comprehensive Cancer Network (NCCN) 2021 suggests performing SLNB for the uterine-confined tumour, with a level of evidence 2A14.
The purpose of this study was to analyse the performance of SLNB using assisted fluorescence imaging of the ICG in patients with early EC, concerning detection rate, sensitivity and negative predictive value (NPV).
Methods
Patients
From September 2019 to September 2022, a retrospective observational analysis of prospectively collected data in a university-affiliated teaching hospital was performed. Patients with clinical stage I or II histologically confirmed endometrial cancer, who underwent SLN mapping, were included. Patients with synchronous cancer were excluded.
All patients with histologically confirmed EC underwent preoperative evaluation with Magnetic Resonance imaging (MRI) of the pelvis to assess myometrial and cervical invasion. Based on MRI and histopathological results, patients were preoperatively stratified into three risk groups for recurrence (low, intermediate, and high), as defined by the European Consensus (2016) 22 and the Portuguese Guidelines on Gynaecological Cancer (2020) 23. High-risk group patients also underwent positron emission tomography scan preoperatively to exclude extrauterine disease.
The study was approved by the local institutional Clinical Research and Ethics Committee, with the number 033-2022.
Surgery
Surgery consisted of laparoscopic SLN mapping followed by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy in all patients. Preoperative intermediate and high-risk patients were also submitted to pelvic ± para-aortic lymphadenectomy up to the level of the renal veins and omentectomy in serous EC and carcinosarcoma, by laparotomy or laparoscopy. Systematic LND was skipped in low-risk patients, based on pre-operative evaluation by MRI, or due to low performance status or high surgical risk.
For the SLNB, ICG was used as the tracer, which is provided as 25mg of sterile powder. The powder was diluted in 10 mL of sterile water. Then, prior to the insertion of the manipulator and the beginning of the laparoscopy, ICG solution was slowly injected into the cervix, with a 22-gauge needle, at the 3 and 9 o’clock positions, superficially (3 mm) under the mucosa and deeply (1 cm) in the cervical stroma. At each moment, 0,25 mL of solution was injected, for a total of 1 mL in a concentration of 2,5 mg/mL.
Intraoperatively, fluorescence detection was performed using the EleVision™ infrared Platform, Medtronic®. After peritoneal evaluation, NIR was activated to identify the tracer in the lymphatic channels, leading to one or more lymph nodes. The identified SLNs were dissected, removed into an endobag and fluorescence confirmed.
Histopathologic Evaluation
All SLNs underwent pathological examination using the ultrastaging technique. Each SLN was sliced at 2 mm intervals and embedded in a paraffin block. Four paraffin-embedded slides were created from each section, 250 µm apart. The slides were stained with Hematoxylin and Eosin, and when negative, analyzed through immunohistochemistry for pan-cytokeratin AE1/AE3 (Dako).
Metastatic disease was classified according to American Joint Committee on Cancer definitions in: isolated tumour cells (single cells or clusters measuring ≤ 0.2 mm), micrometastasis (focus of disease measuring 0.2-2 mm) and macromestastasis (tumour burden > 2 mm) 24.
Statistical analysis
The primary outcome was the SLN detection rate (DR), defined as the detection of SLNs in at least one hemipelvis. The secondary outcomes included bilateral DR, the evaluation of sensitivity, false negative rate, and NPV.
Patients who had mapping of at least one sentinel lymph node were included in the sensitivity and NPV analysis (per protocol). The sensitivity was defined as the proportion of patients with node-positive disease who had metastatic disease correctly identified in SLN. The NPV resulted of the proportion of negative SLN for metastatic disease associated with negative non-sentinel lymph node specimens.
Regarding the comparative analysis of lymph node involvement, each patient served as her own control regarding SLNB and lymphadenectomy results.
All statistical analyses were performed using IBM SPSS Statistics V.26. The comparative analysis was performed using Fisher’s exact test for categorical variables and by Student’s T-Test, Mann Whitney and Kruskal-Wallis tests for continuous variables, according to the normality test results. Statistical significance was defined as p<0.05.
Descriptive data, including demographics, comorbidities, operative, postoperative, and pathologic findings, are presented as mean ± standard deviation (SD) and as median, [interquartile range (IQR), minimum - maximum], according to normality test results, for continuous variables and as frequency (percentage) for categorical variables, respectively.
Results
During the study period, 59 patients with a diagnosis of endometrial cancer apparently confined to the uterus were evaluated for SLN mapping. Three patients with synchronous cancer (renal, ovarian, and lymphoma) were excluded, leaving 56 cases that met the inclusion criteria.
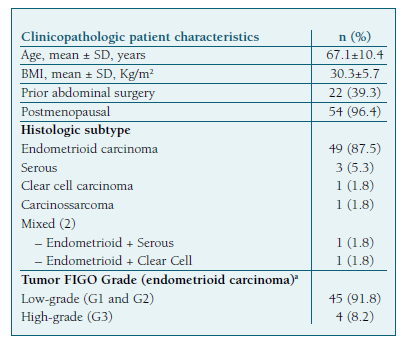
The mean patient age was 67.1±10.4 years and the mean body mass index (BMI) 30.3±5.7 kg/m2. Two patients (3.5%) were premenopausal and 22 (39.3%) had at least one previous abdominal surgery. The clinical and histopathological characteristics of the patients are described in Table I.
Among the included patients, pre-operative risk of recurrence was defined as low in 34 cases (60.7%), intermediate in 14 patients (25.0%), and high-risk in 8 cases (14.2%).
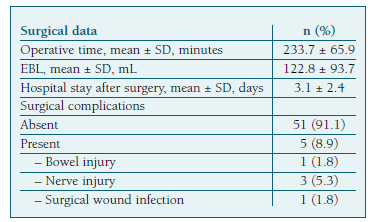
The mean operative time was 233±65 minutes and the mean estimated blood loss (EBL) 122±93mL. (Table II) Forty-five patients (91.8%) had low-grade endometrioid EC and eleven (19.6%) had high-grade cancer. (Table I) Most patients (n=47, 83.9%) were diagnosed as stage IA or IB on the final pathology report. (Table III).
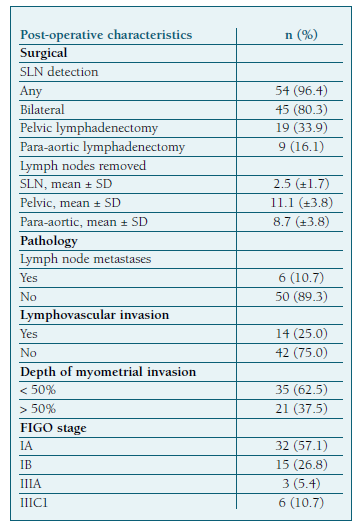
After SLN mapping, systematic pelvic LND was performed in 19 (33.9%) patients and para-aortic LND in 9 (16.1%) (Table III). Omentectomy was additionally performed in five patients.
Mapping identified at least one SLN in 54 (96.4%) patients. Bilateral mapping was achieved in 45 (80.3%) cases and unilateral in 9 (16.1%). Of the 9 cases with unilateral mapping, 2 patients had pelvic LND, another 2 had pelvic and para-aortic LDN, and 5 had only unilateral SLN sampling without contralateral lymph node evaluation. The latter 5 cases belong to the low-risk recurrence group according to pre-operative evaluation. Two cases with negative bilateral mapping were submitted to pelvic LDN. None of them had LN metastasis.
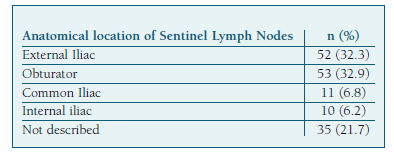
Overall, 161 SLN were detected and retrieved, with a mean (±SD) number per patient of 2.8 (±1.7). The most frequent location (Table IV) was the obturator fossa (32.9%), followed by the external iliac (32.3%).
Metastatic lymph node involvement was identified in 6 cases (10.7%). Low-grade endometrioid EC was the histologic subtype in 5 cases. One patient had high-grade disease. Four of them were in the low-risk preoperative group and had only bilateral SLN mapping. Two patients had bilateral SLN mapping followed by pelvic and paraaortic LDN in one case. The lymph node histopathological evaluation revealed micrometastases in 4 patients and macrometastases in 2.
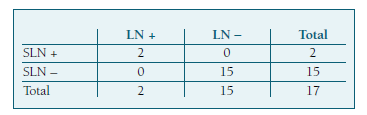
Seventeen patients underwent both SLNB and systematic pelvic and/or para-aortic lymphadenectomy. Two of them had LN metastasis. In one case, the SLN was the only positive node, and in the other one, metastases were identified both in SLN and in pelvic LDN. This leads to a sensitivity and NPV of 100%, with a 0% of false negative rate (Table V).
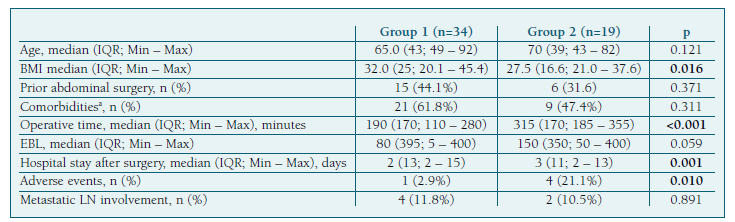
We performed a comparative analysis between the group of 34 patients at low-risk of recurrence who underwent SLN mapping alone (group 1), and the 19 patients from the intermediate and high-risk groups that underwent systematic lymphadenectomy after SLN mapping (group 2) (Table VI). The demographic and clinical characteristics (including age, prior abdominal surgeries and comorbidities) were similar between groups, except for BMI, which was higher in group 1 (median of 32.0 vs. 27.5 kg/m2, p=0.016). We found a significant difference in operative time (median of 190 vs. 315 min, p<0.001) and hospital stay after surgery (median of 2 vs. 3 days, p=0.001), with both being higher in group 2. However, there was no significant statistical difference in EBL between groups. We noted a higher incidence of adverse events in group 2 (21.1% vs. 2.9%, p=0.010). The only surgical complication in group 1 was bowel injury, and there were no adverse effects related to the ICG injection (Table II).
Discussion
This retrospective single-center study aimed to evaluate the accuracy and performance of SLNB using the cervical injection of ICG dye in endometrial cancer patients.
Our results confirmed the high accuracy of this technique, with a high overall and bilateral DR, 96.4% and 80.3%, respectively. Our favorable results are consistent with several other series reported in the literature, with overall and bilateral DR ranging from 86-100%, and 71-90%, respectively12,25,26.
In fact, SLN mapping using the cervical injection of ICG was associated with a higher bilateral DR and similar anatomic lymph node distribution as the hysteroscopic dye injection27 but is easier to perform, making it the recommended alternative to systematic LND by European and NCCN guidelines14,21.
In our study, lymph node metastases were found in 10.7% of the patients, which is consistent with what is reported in the literature6, and all were identified by SLN mapping. No paraaortic SLN were detected in our study, which could be explained by the small sample size. The presence of macrometatic SLN was concomitant with non-SLN metastatic involvement.
Contrary to expected, most of the metastatic lymph nodes were found in a low-risk group (4 of 6 cases), and all of them were classified as micrometastases. These metastases would have been missed if the classic protocol had been followed22. On the other hand, the incorporation of ultrastaging protocol was of great value for identifying the low-volume metastatic disease, considering that 67% of lymph node metastases in our study were micrometastases. All of them were identified through SLNB in presumed low-risk patients, accounting for a prevalence of 11,7% (4/34) of metastasis in this group. In fact, in a study from 2013, Ballester, et al, also identified positive lymph nodes through SLNB in 12,5% of presumed low-risk patients, with detection by ultrastaging of SLN metastases in 42,8% of patients, which would otherwise have gone undiagnosed by conventional histology28.
In our centre, for the purpose of staging and adjuvant therapy selection, micrometastases were considered as lymphatic metastases, resulting in the upstaging of patients and prescription of adjuvant chemotherapy to improve survival rates, as recommended by several studies29,30 and by ESGO, ESTRO and ESP guidelines 202021.
In our study, we achieved a sensitivity and NPV of 100%, with a false negative rate of 0% in a total of 17 cases. This was consistent with multiple previous retrospective and prospective studies, which reported sensitivities of 92.7- 100% and NPV of 99.0 - 100%11,12,25. These results are also in agreement with European Society of Gynaecological Oncology quality indicators for the surgical treatment of endometrial carcinoma31. These findings support the high diagnostic accuracy of the SLNB algorithm for EC in detecting lymph node metastases, sparing unnecessary systematic lymphadenectomies.
The comparative analysis between the low-risk and intermediate/high-risk group showed results similar to those described by Cela V. et al,32 for SLN mapping with ICG in robotic-assisted laparoscopic surgery and suggest that SLN mapping with ICG is a safe procedure that allows quicker surgery with fewer complications and faster recovery for EC patients. Moreover, multiple other studies have suggested a high incidence of intra- and post-operative complications when lymphadenectomy is performed (vs. SLNB) 6,33. The ENDO-3 trial (Phase III Randomised Clinical Trial Comparing Sentinel Node Biopsy With No Retroperitoneal Node Dissection in Apparent Early-Stage Endometrial Cancer - ClinicalTrials.gov NCT04073706) will be the first trial to define the incidence of adverse events, healthcare system costs and health-related quality of life of SLN in the surgical treatment of early endometrial cancer34.
To the best of our knowledge, this is the first study to evaluate the performance of SLNB using ICG in Portugal. We must recognize some limitations of our study, including its small sample size and retrospective single-institution analysis. The strengths rely on the fact that all the surgeries were performed by the same surgical team, minimizing possible discrepancies between different surgeons, and the centralization of all histology samples in the same pathology laboratory, reducing the interobserver bias.
In conclusion, our study provides further evidence to support the clinical application of SLN mapping with ICG cervical injection, as previously demonstrated by other studies. This method appears to be safe and effective in diagnosing lymph node metastases, with a high detection rate and sensitivity. The incorporation of an ultrastaging protocol is crucial for identifying low-volume metastatic disease, which is important for determining definitive staging and adjuvant treatment. In our study, this was especially important for the preoperative low-risk patients’ group.
Authors’ contribution statement
Kristina Hundarova and Cláudia Andrade contributed to the concept and study design. Cláudia Andrade was responsible for the collection of data. Kristina Hundarova was responsible for the analysis and interpretation of data and the article draft. Cristina Frutuoso, Fernanda Águas and Cláudia Andrade supervised the team research and revised the article critically. All authors approved the final article as submitted and agreed to be accountable for all aspects of the work.