Introduction
Malignant melanoma is an extremely aggressive tumor, which is most often found in the skin. More rarely, these tumors can be found in the mucosa of respiratory, gastrointestinal or genitourinary tracts. These mucosal melanomas account for 1% of all melanomas and, they have different etiologies from the ones involving the skin, with unknown risk factors1. Mucosal melanomas are even more aggressive than the skin type of this malignancy. Being much more difficult to diagnose, they are usually detected in advanced stages.
Malignant melanoma of the vagina represents only 3% of all melanomas of the female genital tract (FGT), since more than 95% of mucosal melanomas of the FGT occur in the vulva, and only 0,3% to 0,8% of all melanomas in women1. The incidence is only 0,46 cases per 1 million women, therefore, less than 500 cases are currently reported in the literature2-4. PVMM have an exacerbated tragic behaviour because of their ability to disseminate, with a 5-year survival rate of 0-25%1,6.
The objective of this article is to present a case of PVMM with a brief review of the current literature.
Case Report
A 74-years-old, postmenopausal, caucasian woman, resident in a nursing home, presented with a mass emerging from the vagina, associated with intermittent vaginal bleeding. The patient was completely dependent on caregivers due to an ischemic stroke resulting in a right hemiparesis and aphasia. She did not have any family history of malignancy.
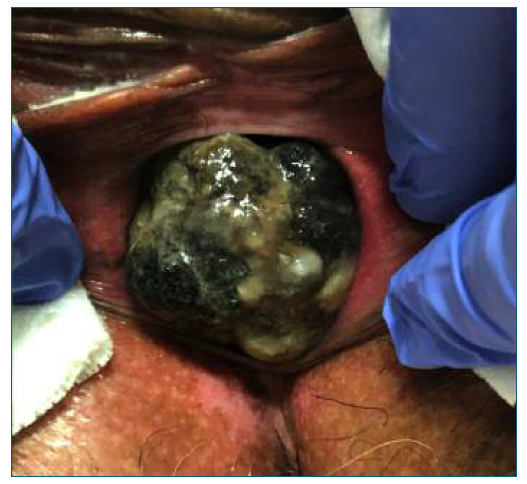
Vaginal examination showed a 4 cm brown-colored solid tumor, arising from the inferior third of anterior vaginal wall. No other lesions or vaginal changes were identified. This lesion was necrotic and bled to the touch. It appeared to be continued with urethral posterior wall, but with external growth, without obstruction of the urinary tract. Bilateral parametria were free and rectum was normal, and there was no palpable inguinal lymphadenopathy. Total-body skin and ocular examination ruled out primary cutaneous or ocular melanoma. A directed punch biopsy of the lesion revealed a PVMM.
Magnetic resonance imaging (MRI) of the pelvis demonstrated a 5,3×4×3,7 cm expansive lesion, invading the urethra back wall, with extension to the urethral meatus, but without suspicious lymph nodes. MRI of the brain and Computerized Tomography (CT) of the chest, abdomen and pelvis, exhibited a small and indeterminate hepatic lesion, however, definitive evidence of metastasis was not histopathologically confirmed by biopsy. Positron Emission Tomography (PET) confirmed a large hypermetabolic lesion in the vagina and no signs of lymphadenopathy or distant metastases.
Because of the patient’s medical condition, she did not fulfilled criteria for surgical intervention. She also refused systemic treatments, radiotherapy or immunotherapy. Six months later, she presented with a pathological fracture and, CT scan revealed hepatic and bone lesions suggesting distant metastases. Unfortunately, the patient died a few weeks after.
Discussion
The majority of malignant melanomas are cutaneous, hence finding mucosal melanomas are uncommon. Furthermore, in the FGT, vulvar melanomas are exceedingly more frequent, consequently, vaginal melanoma is an exceptionally rare entity2,7.
PVMM, by definition, should be originated from the vaginal wall, without engagement of vulva or cervix, and not resulting from a distant metastasis of any other primary tumor8. The most common site of presentation is the lower third of the anterior vaginal wall, as well as in our case5,7.
Literature suggests that these tumors arise from melanocytes, present in the basal layer of the vaginal epi-thelium in 3% of women as an embryological remnant of neural crest cells1. The role of melanocytes present in the skin is well known - protection from sun-exposure through the production of melanin. On the other hand, the function of melanocytes of the mucosa lining the FGT is obscure. However, some authors question if these cells might have an antimicrobial and immunological activity1.
The etiopathogenic pathway for mucosal melanomas is poorly understood. Risk factors are unknown and genome sequencing appears to be unable to find ultraviolet related or cigarette smoking specific mutational patterns7. Myriad somatic mutations have been identified among cutaneous melanomas, most commonly in BRAF, representing up to 62% of all cases, NRAS (10-28%) and NF1 (14%)7. In contrast, approximately 55% of mucosal melanomas are wild type for these oncogenes. Up to 39% will have c-KIT mutations, 12% NRAS mutations and 9-19% BRAF mutations7. Molecular analysis of vulvar and vaginal melanoma has not presented significant variations, except for c-KIT mutations9.
Further analysis of the genetic differences between mucosal and cutaneous melanomas is needed to understand the causes of mucosal melanomas and to enhance the role of immunotherapy and targeted therapy in the treatment of PVMM.
This type of mucosal melanomas mainly affects post-menopausal women, with an average age of 60 to 70 years10. The most common form of presentation is vaginal abnormal bleeding (63,6%-80%) and, usually it can only be diagnosed with a speculum examination. Less frequent symptoms are vaginal mass (15%-15,9%), vaginal pain (2,3-10%), vaginal discharge (15,9%-25%), dyspareunia or pruritus6,11. A large part of women at this age does not accomplish a routine gynecological examination, as a result, 28 to 50% have advanced disease at diagnosis7,12,13. In our case, the patient was a disabled woman living in a nursing home without gynecologic monitoring, which led to the exponential growth of the vaginal mass, and to a delayed diagnosis.
PVMM might have different shapes, such as solitary, multifocal, superficial spreading and nodular6. More frequently they are pigmented, polypoid or nodular lesions and often ulcerated4,10. However, 10 to 23% are amelanotic and, for that reason, highly similar to vaginal epithelial tumors4. The differential diagnosis is mainly between poorly differentiated squamous cell carcinoma, metastasis from other tumors, sarcoma, lymphoma and benign blue nevus10.
The diagnosis is established by histological and immunohistochemical examination, with positive staining for protein S-100, melan A, human melanoma black 45 (HMB-45) and vimentin, as well as evidenced in cutaneous melanomas4,15. Tumor cells could be epi-thelioid - most frequently, spindled or mixed type16. In histopathologic analysis, tumor depth, presence of ulceration, epithelioid cell type, presence of microsatellites, angio-lymphatic involvement, high mitotic rate, amelanosis and development from a pre-existing nevus, are considered poor prognostic factors17.
Radiologic evaluation is essential for tumor staging. Accordingly, pelvic MRI is important to diagnose locoregional extent, CT-scan and PET are particularly useful on detecting metastatic disease3,4. In terms of clinical staging of PVMM, International Federation of Gynecology and Obstetrics (FIGO) guidelines for vaginal tumors could be applied, however, it does not consider tumor size nor regional lymph nodes status5. Tumor size is indicated as the most important prognostic factor - tumors less than 3 cm, have a survival rate of about 41 months; larger than 3 cm, about 12 months of survival4. Some authors consider American Joint Committee on Cancer’s (AJCC) TNM method, used on skin cancer lesions, more reliable in terms of prognostic prediction4,18. Others, contemplate the use of both classification systems in association4,19.
The severity of this tumor, which makes it the most dangerous form of vaginal tumors, is related to its capacity for early recurrence, invasion of locoregional lymph nodes and hematogenous dissemination, resulting in distant metastases in the lungs, liver, bones and brain. Additionally, this tumor may cause life-threatening vaginal hemorrhage. At diagnosis, the majority of patients have advanced disease, with 50% having positive regional lymph nodes and almost 20% having distant metastases5. This aggressive capability to spread can be explained by the extensive lymphatic and vascular supply to the lamina propria of the vaginal mucous membranes3. The most frequent locations for early recurrence are the vagina, vulva and groin3. In our case, the lower third of anterior vaginal wall was affected.
Another challenging aspect of PVMM is the treatment. As a result of its rarity, standard protocols aren’t available. Regarding the surgical approach, when the melanoma can be resected, this is always the preferred treatment3,4,6,19. There are two possible modalities: conservative surgery, with wide local excision; or radical surgery - vaginectomy with or without vulvectomy and, in more advanced cases, pelvic exenteration. Literature advocates conservative approach and radiotherapy as the preferred strategy3,5. In more advanced stages, patients can undergo adjuvant radio-chemo-therapy after radical surgery4. If lymph node metastasis is not present, lymphadenectomy should not be performed4. Radiotherapy may also be offered when patients are unable or unwilling to undergo surgery, as in our case5. Chemotherapy regimens are essentially used as palliative treatments because they failed to prove beneficial in randomized controlled trials3. Furthermore, immunotherapy with Interferon alpha and targeted therapy have been showing promising results in metastasized cutaneous melanomas, nonetheless, additional studies in mucosal melanomas are needed3,4,20.
In conclusion, PVMM is a rare form of mucosal melanomas, very unusual and much more aggressive than vulvar melanomas. The malignancy has a very poor prognosis because of its delayed diagnosis; therefore, frequent gynecologic evaluation and biopsy of every suspicious mass are recommended. The role of adjuvant therapies continues undefined since prospective studies need to be performed to ensure its benefits17. Due to the limited number of cases reported in the literature, sufficiently enpowered evidence is lacking, so the development of guidelines for the treatment of PVMM is a challenge.
Authors’ contributions
Ana Cláudia Lopes: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft. Mariana Santos: formal analysis, investigation, writing-review & editing. Eliana Teixeira: supervision, validation, writing-review & editing. Vera Vilhena: supervision, validation, writing-review & editing. Marilia Paizinho: resources, supervision, validation.