Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Angiologia e Cirurgia Vascular
Print version ISSN 1646-706X
Angiol Cir Vasc vol.15 no.2 Lisboa June 2019
ARTIGO DE REVISÃO
Endovascular treatment of chronic venous occlusive disease - specifications of endoprostheses and comparison of results
Tratamento endovascular da doença venosa crónica oclusiva - especificações das endoproteses e comparação de resultados
Daniela Bento1, Rui Machado1,2, Daniel Mendes2, Rui de Almeida1,2
1 Instituto de Ciências Biomédicas Abel Salazar - Universidade do Porto
2 Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar Universitário do Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Chronic occlusive venous disease manifests mainly at the level of the iliofemoral vein, and its treatment has been revolutionized by the emergence of endovascular techniques. Venous system stenting has evolved from the existing treatments of arterial occlusive disease. Some arterial stents were used in the venous system with good results, however, the need to improve the characteristics of these devices led to the development of stents dedicated exclusively to venous pathology. In recent years several dedicated endoprostheses have been approved, however, there are few studies comparing their characteristics and results.
Tratamento endovascular da doença venosa crónica oclusiva - especificações das endoproteses e comparação de resultados
Objectives: Update on the venous stents available and comparison of their characteristics and results.
Methodology: The bibliographic research was performed in database "Natural Library of Medicine PubMed - Medline". Articles from the last 20 years with language in Portuguese and English were included. Greater relevance was given to research articles, but books and review articles relevant to the topic were also included
Results/Discussion: There are currently 7 devices used in iliofemoral occlusive disease Wallstent" Endoprosthesis, Zilver® Vena ", Sinus-Venous®, Sinus Obliquus®, Vici® Venous Stent, Venovo® Venous Stent, Sinus Obliquus®and Abre" Venous. The short-term outcomes show high rates of technical success, primary and secondary patency, null mortality and low rates of periprocedural complications.
Conclusion: Existing stents for venous use appear to be effective and safe in the treatment of iliofemoral occlusive venous disease. None of the devices stand out in terms of effectiveness, however, dedicated stents appear to have lower complication rates. Long-term studies are needed to confirm these results.
Keywords: Venous insufficiency, Venous thrombosis, Self-Expandable Metal Stents, Endovascular procedures
RESUMO
Introdução: A doença venosa crónica oclusiva manifesta-se principalmente ao nível do setor íleo-femoral, e o seu tratamento tem sido revolucionado pelo surgimento das técnicas endovasculares. O stenting do sistema venoso evoluiu da experiência existente no tratamento da doença oclusiva arterial. Alguns stents arteriais foram utilizados no sistema venoso com bons resultados, no entanto, a necessidade de aprimorar as características destes dispositivos levou ao desenvolvimento de stents dedicados exclusivamente à patologia venosa. Nos últimos anos várias endopróteses dedicadas foram aprovadas, no entanto, há poucos estudos que comparem as suas características e resultados.
Objetivos: Atualização sobre os stents venosos disponíveis e comparação das suas características e resultados.
Metodologia: Pesquisa bibliográfica realizada na base de dados “Natural Library of Medicine PUBMed - Medline”. Foram incluídos artigos dos últimos 20 anos com idioma em português e inglês. Foi atribuída maior relevância a artigos de investigação, no entanto, foram também incluídos livros e artigos de revisão com interesse para o tema.
Resultados/Discussão: Existem atualmente 7 dispositivos usados na doença oclusiva iliofemoral Wallstent "Endoprosthesis, Zilver® Vena", Sinus-Venous®, Vici® Venous Stent, Venovo® Venous Stent, Sinus Obliquus® e Abre " Venous. Os resultados apresentam altas taxas de sucesso técnico, patência primária e secundária a curto prazo, mortalidade nula e baixas taxas de complicações periprocedimento.
Conclusão: Os stents existentes para uso venoso parecem ser eficazes e seguros no tratamento da doença venosa oclusiva iliofemoral. Nenhum dos dispositivos se destaca em termos de eficácia, no entanto, os stents dedicados parecem apresentar taxas de complicação mais baixas. São necessários estudos a longo prazo para confirmar estes resultados.
Palavras-chave: Insuficiência Venosa, Trombose venosa, endopróteses venosas, procedimentos endovasculares
Introduction
Chronic Venous disease
Chronic venous disease (CVD) is a pathology of the vascular system with high prevalence amongst the adult population, averaging a world-wide prevalence of approximately 83%.(1)
CVD has a great socio-economic impact, not only due to the high number of affected individuals, their diagnosis and treatment costs, but also due to the debilitating and painful effects of the disease, which are reflected on loss of ability to work and overall poor quality of life.(2,3) In Portugal, CVD affects approximately a third of the population.(3)
The clinical presentation of CVD is frequently associated with discomfort of the lower limbs. Clinical signs might include telangiectasia, reticular veins, varicose veins, edema and altered pigmentation of the skin. Venous claudication and venous ulcers occur in severe cases.(4,5)
Given the great variability of clinical presentations, a classification system was created to standardize the diagnosis and staging of CVD, the CEAP system. This system grades CVD from C0 to C6, based on increasing severity of the condition.(6)
The main predisposing factors for CVD are age (older individuals are more predisposed), number of pregnancies, gender (females are at higher risk), overweight and family history of the condition.(3,7,8) Other factors, such as sedentary life-styles, smoking, frequent constipation and long periods standing or seating also seem to have some degree of influence on the onset and progression of the pathology.(7,9)
The pathophysiology of CVD is based on venous hypertension. This can in turn originate either by venous reflux, allowing the retrograde movement of blood, or by venous obstruction, which is characterized by a mechanical block to the normal blood flow. These mechanisms can act separately or in association, having a synergetic effect that produces worst clinical presentations.(5,7,10)
Valvular incompetency is the main cause of venous reflux and can be due to pre-existing weakness of the venous wall or valvular sheets, or due to damage caused by phlebitis or deep venous thrombosis.(4,10)
Deep veins, such as the vena cava, iliac or femoral veins are mostly affected by obstructions, and can be classified based on their etiology as primary obstruction when unrelated to thrombosis and secondary obstruction when related to a thrombosis. From an anatomic point of view, the obstruction can be classified as intrinsic when is caused by a thrombus or stenosis, or extrinsic when there is extra-mural compression, from a neoplastic lesion or May Thurner.(5,7,9,10)
Th first line of treatment of CVD of the lower limbs is a conservative approach, based on compressive therapy. This can be supported with other forms of therapy such as physiotherapy, lymphatic drainage and venotropic drugs. Frequently the conservative approach is not enough, and invasive procedures are necessary.(9,11)
The main focus of an endovenous surgical therapy used to be correcting the reflux, removing or obliterating incompetent veins and isolating the origin of the reflux from the vascular system.(4,5,7,10,11)
With the evolution of the diagnostic techniques and wider knowledge of the occlusive etiology, the treatment modalities were adapted to address this form of CVD as well.
Chronic Occlusive Venous Disease
Chronic occlusive venous disease (COVD) is most frequently present at the iliofemoral segment.(9) As previously mentioned, this occlusion can have different etiologies, but is mainly referred to as non-thrombotic (NT) occlusion or post-thrombotic (PT) occlusion, in order to simplify its classification.(5,7,9,10)
The surgical approach to this condition was based on vascular reconstruction, usually as a surgical bypass, associated with high mobidity. The technological advances on the endovascular treatment allowed for minimally invasive procedures to take over the previously used techniques, improving the overall results.(11-13) By being minimally invasive and having a lower mobidity, angioplasty with stents have been the most commonly used technique.(11-13)
The treatment of COVD has proven to have good results at relieving the symptoms of CVD, even in patients with concomitant reflux disease, and has, therefore, been suggested by many authors as first line treatment for CEAP grade 3 and above, always when there has been found occlusion.(11-14)
Venous stenting
The stenting of the venous system emerged in the early 1990s, having evolved from experience and existing devices for the treatment of occlusive disease of the arterial system and biliary tract, however the characteristics of diseases and vessels are quite different (12,15,16)
While the main etiology of arterial disease is atherosclerosis, in COVD, vessel obstruction is due to venous thrombosis or external compression. Venous blood pressure is lower and the mechanical stress points are different from arterial ones.(12) Veins behave differently than arteries due to higher elastic recoil. Elastic recoil refers to a rebound of the vessel wall after percutaneous transluminal angioplasty that results in recurrent narrowing. This is especially relevant for venous lesions of iliac and central veins, described to have high elastic recoil. Due to this process, patency after isolated angioplasty of iliac veins is poor and almost all patients will require a stent to treat COVD.The iliofemoral veins are subject to repeated trauma by the pulsation of adjacent arteries, and subject to continuous deformations due to pelvic mobility during ambulation. Some anatomical points such as the iliac bifurcation, the iliocaval junction and the posterior area to the inguinal ligament are external compression points that may condition fibrosis and luminal alterations.(12,15)
These differences must be considered when choosing a stent and the device must have physical and mechanical properties which allows appropriate adaptation to the venous system environment. Therefore, to obtain a good performance, the stent must present:
High Radial Force
Radial force is defined by the pressure that the stent exerts on the vessel during expansion, which allows a good placement of the stent against the wall of the vessel. This property is important as it reduces the migration of the device(16-18) 18 In venous circulation, blood pressure is lower, which causes less circumferential parietal stress, and therefore a greater radial force is required to anchor the stent at the desired level. This increase in radial force can be achieved by using devices with a larger diameter than the vessel. Due to these conditions, the diameter of venous stents is usually larger than the diameter required for the arterial system.(12,15)
High Radial Resistance
Radial resistance is defined as the radial compressive strength capacity of the stent. Stent strength is an important quality in anatomical stress points, but also necessary to overcome luminous changes such as fibrosis and adhesions. Radial resistance is important to overcome stent compression, one of the most frequent causes of chronic stent malfunction. Stent compression occurs exclusively in the venous system, wherein the stent is compressed from the outside, reducing the lumen of the vessel and is caused by fibrosis/restenosis of the stented segment.(12)
Good flexibility
Flexibility allows the stent to adapt to the shape of the vein and to the change in pelvic geometry with ambulation, without bending or significant reduction in the cross-sectional area.(17) Current stents made for the arterial system are often quite rigid. Stiffness may lead to non-conformity between stent and anatomic alterations of the vein.(12,17)
Minimal foreshortening
Minimal retraction of the stent allows for precise placement of the device, without any subsequent change in its size.(17)
High Durability
Patients with COVD are relatively younger than those suffering from atherosclerosis, so a venous stent should be considerably longer lasting (around 50 years). The material should be resistant to corrosion and fatigue and long-term stent stenosis/thrombosis should not occur(12,17)
Optimized structure and design
Vascular stents usually consist of Z-shaped sequential rings (called struts), which are interconnected by bridges or hinges. Variations in these interconnections give rise to different types of "cells", and stents are characterized according to their design in closed-cell and open-cell.(19)
In closed-cell stents, all sequential rings are interconnected by bridges.(19,20) The main advantages of these devices are the uniform surface and the optimal scaffolding provided, but their flexibility is more limited.(19)
In open-cell stents the interconnections are punctual and scarcer, which ensures greater flexibility and less foreshortening, however, the structure of the device becomes less strong and less resistant.(19)
The stent design influences the contact area between the device surface and the vessel. This contact should be minimal in order to reduce the thrombotic response to the stent material.(17)
High Biocompatibility
The material should not cause adverse reactions to the bearer..(17)
Radiopacity:
Visibility of the device in fluoroscopy is required for implantation and subsequent patient follow-up. However, at the same time, the material should cause minimal artefact on imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI), allowing a good evaluation of adjacent structures..(12)
In order to assess the safety and effectiveness of a stent, it is important not only to know its characteristics, but also to assess its short and long term results.(9,16,18)
In recent years, with the increasing use of the endovascular technique in CVOD, several stents have been developed specifically for the venous system, however, there are few studies that compare its characteristics and results.(15,16,18)
Objectives
The objective of this review is to provide an update on the available venous stents and to compare their characteristics and results.
Materials and Methods
Bibliographic research conducted in the database "Natural Library of Medicine PUBMed - Medline". The keywords used were the MeSH terms: "Venous Insufficiency", "Venous Thrombosis", Self-Expandable Metal Stents" and "Endovascular Procedures". The search for articles was limited to the last 20 years in Portuguese and English language. The selection and exclusion of articles was based on the title and the abstract containing information on primary patency, primary assisted patency, secondary patency and periprocedural complications. More emphasis was given to research
Results/ Discussion
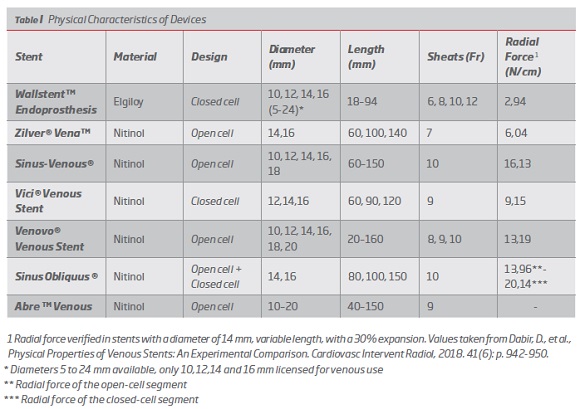
1. Physical characteristics of devices
The stents currently available for endovascular treatment of iliofemoral COVD are as follows:
Wallstent" Endoprosthesis (Boston Scientific, Marlborough, USA)
This device was developed more than 20 years ago and was initially designed for intervention in the biliary tract and then adapted for tracheobronchial, gastric, and venous use.(16,21) It was approved for use in the venous system by the FDA in 2001 and the CE label in 2015.(21,22) It is a closed-cell self-expandable deployment system composed of elgiloy® metal alloy (Cobalt-Chrome-Nickel-Molybdenum) with braided configuration. The device is available in diameters from 5 to 24 mm, and for venous use only diameters 10, 12, 14 and 16 mm are licensed.(16,19,21) In terms of length there are devices from 18 to 94 mm.(16,22,23) It is compatible with 6-12Fr sheaths and 0.035 inch guide wire. It can be implanted through systems of 75 and 135 cm.(16,19,21) According to Dabir et all, it has a radial force of 2.94 N/cm with 30% expansion, and this value increases to 5.4 when the extremities are fixed.(16) It has a radiopaque body for greater visibility in fluoroscopy.(22) This device is indicated only for central venous use, in patients under hemodialysis that maintains stenosis of the venous outflow tract after unsuccessful angioplasty. However, its off-label use in symptomatic venous obstruction in iliofemoral veins has been very frequent (Post-thrombotic syndrome, recurrent thrombosis of the iliofemoral vein, DVT, May-Thurner syndrome, extrinsic neoplastic compression). (16,22-24) (Table I).
Zilver® Vena" (Cook Medical Technologies, Bloomington, Indiana, USA)
This device was the first to be developed specifically for the venous system.(25) It received CE approval in 2010 and is currently under study for FDA approval.(21,25) It is a self-expandable nitinol stent (nickel-titanium alloy) with open cell design.(16,25,26) The device is available in diameters of 14 and 16 mm and lengths of 60, 100 and 140 mm.(16,25,26) It is compatible with 7Fr sheaths and 0.035 inch guide wire. It can be implanted through systems of 80 and 120 cm.(16,25,26) It has 4 marks at each end for greater visibility. According to Cook Medical, the radial force is 30% higher than the Zilver® predecessor, and one study evaluated its radial force at 6.04 N/cm with 30% expansion.(16,25,26) The use of this device is indicated for symptomatic venous obstruction in iliofemoral veins.(16,25,26) (Table I).
Sinus-Venous® (OptiMed GmbH, Ettlingen, Germany).
This device appeared in 2012 and was the second stent dedicated to the venous system to obtain CE approval. It is not approved for venous use by FDA(21,25) It is a self-expanding nitinol stent with a combined open cell design, consisting of independent rings interconnected at 2 points by metal bridges called "Flash Links".(16,27,28) The device is available in diameters from 10 to 18 mm and lengths from 60 to 150 mm. (16,27,28) It is compatible with 10Fr sheaths and 0.035 inch guide wire. It can be implanted through systems of 100 cm. (16,27,28) It has a maximum radial force of 16,13 N/cm with expansion at 30%.(16) It has radio markers at the ends for greater visibility and is licensed for treatment of symptomatic venous obstruction in the iliofemoral veins.(16,27,28) (Table I).
Vici® Venous Stent (Veniti, Inc. / Boston Scientific Fremont, California, USA)
This device is designed for venous use. It received CE marking in 2013 and was also recently approved by the FDA - May 2019.(29) Vici Venous is a self-expandable nitinol stent with closed cell design with sinusoidal support rings and alternate bending bridges.(12,16,29-31) The device is available in diameters of 12, 14 and 16 mm and lengths of 60, 90 and 120 mm.(12,29) It is compatible with 9Fr sheaths and 0.035-inch guide wire.(12,29) It has a radial force of 9.15 N/cm with 30% expansion.(16) The use of this device is indicated for symptomatic venous obstruction in iliofemoral veins.(12,16,30-32) (Table I).
Venovo® Venous Stent (Bard, Tempe, USA)
This venous stent received CE marking in 2014 and it was also recently approved by the FDA in March 2019.(16,33-35) It is a self-expanding nitinol stent with open cell design.(16,33,35) The device is available in diameters from 10 to 20 mm and lengths from 20 to 160 mm.(12,35) It is compatible with 8, 9 and 10Fr sheaths and 0.035 inch guide wire. It has 6 radiopaque marks on each end for better visibility.(35) It has a radial force of 13.96 N/cm with 30% expansion.(16) The use of this device is indicated for symptomatic venous obstruction in iliofemoral veins.(16,33-35) (Table I).
Sinus Obliquus ® (OptiMed GmbH, Ettlingen, Germany).
This device was specifically developed for iliac vein obstructions near the iliocaval junction(12,16) Its use has been approved by the CE since 2015. It is not FDA approved for venous use.(21) Sinus Obliquus is a self-expandable nitinol stent with hybrid conformation since it features a closed cell design that provides high radial force at the compression site and distally an open cell design to provide greater flexibility needed for ambulation and better fit to the curved anatomy of the iliac vein.(36) It also presents an oblique cut (35°) in the proximal region, whose objective is to avoid protrusion of the stent into the inferior vena cava, which could compromise the blood flow of the contralateral iliac vein.(36) The device is available in diameters of 14 and 16 mm and lengths of 80, 100 and 150 mm.(12,16) It is compatible with 10Fr sheaths and 0.035 inch guide wire. It has 4 marks on the proximal end for greater visibility. The maximum radial force verified for this device is 13.96 N/cm in the distal segment (open cell) and 20.14 N/cm in the proximal segment (closed cell) with an expansion of 30%.(16) (Table I).
Abre" Venous Stent System (Medtronic, Minne-apolis, USA).
This device is the latest on the market. It has been CE approved since April 2017, and the ABRE IDE study is in progress for FDA approval.(21) It is a self-expandable nitinol stent with an open cell design with 3 connection points between the cells.(12,16,37) The device is available in diameters from 10 to 20 mm and lengths from 40 to 150 mm. It is compatible with 9Fr sheaths and 0.035-inch guide wire.(16) It can be implanted through 90 cm systems. The use of this device is indicated for symptomatic venous obstruction in iliofemoral veins.(12,16,37) (Table I).
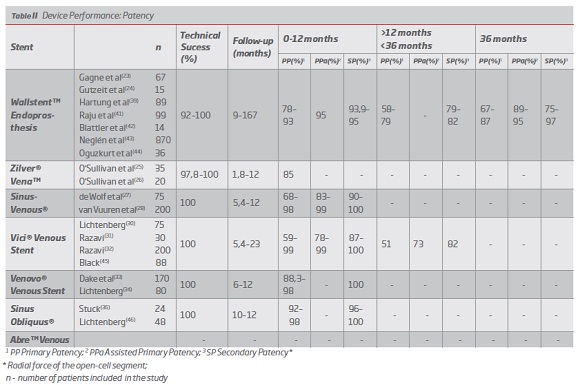
2. Device performance
Device performance was characterized by the analysis of the following variables: technical success, primary patency (PP), assisted primary patency (PPa), secondary patency (SP), periprocedural complications (including stent fracture, contralateral iliac vein occlusion, stent occlusion, restenosis, reintervention, migration and mortality).
It is considered technical success when stent implantation allows for the restoration of the obstructed vessel.(9,23,24) The definitions of primary patency (uninterrupted patency without intervention in the device), assisted primary patency (after prophylactic intervention in a non-occluded device) and secondary patency (restored patency after device occlusion) considered were those indicated by Rutherford in 1997.(38)
Abre" Venous stent’s performance, although previously mentioned, will not be characterized due to the lack of support literature.
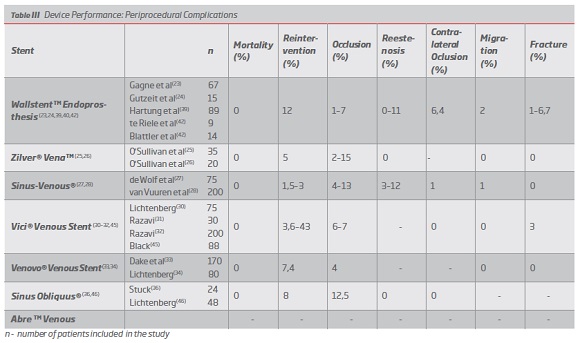
Wallstent was the first device to be used in the treatment of COVD, initially off-label, and for this reason it is the stent for which there are more studies and more clinical experience. (9,12) The studies for this stent present a mean follow-up of 9 to 167 months, with short, medium and long-term results.(23,24,39) The technical success is high with values between 92 and 100%. In the short term (between 0 and 12 months) this device presents PP between 78-93%, PPa 95% and SP between 93.9-95%.(37,40) In the medium term (more than 12 months and less than 36 months) it presents PP values between 58-79% and SP values between 79-82%.(41,42) In the long term (more than 36 months) it presents PP values between 67-87%, PP 89-95% and SP values between 75-97%.(23,39,43,44) (Table II) In terms of complications, the following results were found: 0% mortality, 12% reintervention, 1-7% stent occlusion, 0-11% stenosis, 6.4% contralateral occlusion, 2% stent migration and 1-6.7% stent fracture. (23,24,39,40,42) (Table III).
Regarding the Zilver Vena stent, although it was approved in 2010, there are not many studies that assess its performance. A randomized clinical trial is currently underway in the US - VIVO Clinical Study - whose results are not yet available. Existing studies have an average follow-up between 1.8 and 12 months, so there are only short-term results. The technical success is high, between 97.8 and 100%. In the short term (between 0 and 12 months) this device presents PP between 85 and 87.9%, with no data on PPa or SP. (Table II) In terms of post-implantation complications, the following results were found: 0% mortality, 5% reintervention, 2-15% stent occlusion, 0% restenosis, 0% stent migration and 0% stent fracture. None of the studies referred to contralateral occlusion.(25,26) (Table III)
Optimed's Sinus Venous has an average follow-up between 5,4 and 12 months, which also limits the assessment of its performance in the short term. The technical success is 100% in all articles analyzed. In the short term (0 to 12 months), this device presents PP between 68-99%, PPa between 83-99% and SP between 90-100%. (Table II) In terms of periprocedural complications, the following results were found: 0% mortality, 1.5-3% reintervention, 4-13% stent occlusion, 3-12% restenosis, 1% contralateral occlusion, 1% stent migration and 0% stent fracture.(27,28) (Table III).
The Vici Venous device from Viniti / Boston Scientific, approved by the FDA and CE, presents a mean follow-up between 5,4 and 23 months, being the first of the dedicated stents to present results in the medium term. The technical success of this stent is 100%. In the short term (0 to 12 months), this device presents PP between 59-99%, PPa between 78-99% and SP between 87-100%.(30-32) In the medium term (more than 12 months and less than 36) it presents PP of 51%, PPa 73% and SP of 82%.45 (Table II) The following rates of periprocedural complications were found: 0% mortality, 3.6-43% reintervention, 6-7% stent occlusion, 0% stent migration, 3% stent fracture, 0% contralateral occlusion, and no data on restenosis were reported.(30-32,45) (Table III)
The Venovo device, approved by the FDA and CE, has an average follow-up of 6 to 12 months. The technical success of this stent is 100%. In the short term (0 to 12 months), this device presents PP between 88,3-98%, PPa between 94-99% and SP of 100%. (Table II) The following rates of post-implantation complications were found: 0% mortality, 7.4% reintervention, 4% stent occlusion, 0% stent migration and 0% stent fracture, and no data on stenosis or contralateral occlusion were reported.(33,34) (Table III).
The Sinus Obliquus device also presents limited literature in the short term, with a mean follow-up of between 10 and 12 months. The technical success is 100%. It presents PP between 92-98% and SP between 96-100%. (Table II) The level of periprocedural complications was as follows: 0% mortality, 8% reintervention, 12.5% stent occlusion, 0% restenosis, 0% contralateral occlusion, with no data on other complications.(36,46 )(Table III).
3. Discussion
From the analysis of the afore mentioned results it is possible to conclude that all the devices show a high technical success rate (over 92%) and high safety, with no reported associated deaths and low complication rate (under 13%). This data is in accordance with the previously published systematic reviews, which report success rates of 94 to 98%, death rate of 0,3 to 1,1% and complication rates of 0 to 8,7%.(9,47-50)
The main complications associated with the use of the Wallstent during the initial period of application to the venous system were contralateral iliac vein obstruction, fracture and migration of the stent.(12) These complications are related to certain technical challenges as foreshortening and lessened radial force at the ends when not restrained. The need to overcome these complications motivated the development of dedicated stents devices.(23) By analyzing the results of this paper it is possible to find lower complication rates associated with the use of the venous specific stents, which makes a strong argument for their use (contralateral occlusion 6.4% vs 0 -1% , fracture 1-6,7% vs 0-3%, migration 2% vs 0-1%).
The occlusion of the device is the most frequently reported complication and it is present across the whole range of devices reviewed. This rate is highly influenced by the thrombotic or non-thrombotic etiology and severity of the primary condition, more so than by the characteristics of the device used.(5,9,48,49) According to Razavi(9), the occlusion rate varies between 1 and 6,8%. For the Zilver Vena, Sinus Venous e Sinus-Obliquus stents, some studies show slightly higher values (12-15%).
It is not possible to accurately compare the patency and reintervention rates between the devices given the lack of standardization of the samples of the various published studies and the lack of long-term follow-up of patients with the most recent stents. Nevertheless, reintervention rates are high in practically all stents and are largely due to in-stent restenosis which the pathophysiology is not adequately known. Treatment of this condition usually consists of in-stent transluminal angioplasty or repeat stenting and is associated with high rates of clinical and imagological recurrence.
According to other published relevant reviews, short term and medium term PP and PS are respectively 32-98,7% e 66-96%.(9,49,50) The results of this review are in accordance with the available literature, with PP and PS as high as 100% (Table II), supporting the efficacy of these devices on the short term.
Wallstent is the only device which presents long term follow up results, with high PP and PS, ultimately supporting its long-term efficacy.
Conclusion
The stents available for venous disease seem to be safe and adequate at treating occlusive venous disease of the iliofemoral segment, show high technical success rates and high patency, no mortality and low periprocedural complication rate at short-term follow-up. None of the devices stands out with regards to efficacy, although venous specific devices seem to show slightly lower complication rates. Further studies on the long-term complication rate of the new endoprosthesis are necessary to confirm these results.
REFERENCES
1. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31(2):105-115. [ Links ]
2. Rabe E, Pannier F. Societal costs of chronic venous disease in CEAP C4, C5, C6 disease. Phlebology. 2010;25 Suppl 1:64-67. [ Links ]
3. Fernandes J, Natário A, Matos A, et al. Rede de Referenciação Hospitalar Angiologia e Cirurgia Vascular. In: SNS, ed: Republica Portuguesa; 2017:34-39. [ Links ]
4. Youn YJ, Lee J. Chronic venous insufficiency and varicose veins of the lower extremities Korean J Intern Med. 2019;34(2):269-283. [ Links ]
5. Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: A current comprehensive meta-analysis and systematic review. Phlebology. 2016;31(6):376-389. [ Links ]
6. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248-1252. [ Links ]
7. Attaran RR. Latest Innovations in the Treatment of Venous Disease. J Clin Med. 2018;7(4):1-16. [ Links ]
8. Pannier F, Rabe E. The relevance of the natural history of varicose veins and refunded care. Phlebology. 2012;27 Suppl 1:23-26. [ Links ]
9. Razavi MK, Jaff MR, Miller LE. Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2015;8(10):e002772. [ Links ]
10. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-346. [ Links ]
11. Santler B, Goerge T. Chronic venous insufficiency - a review of pathophysiology, diagnosis, and treatment. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2017;15(5):538-556. [ Links ]
12. Shamimi-Noori SM, Clark TWI. Venous Stents: Current Status and Future Directions. Techniques in Vascular and Interventional Radiology. 2018;21(2):113-116. [ Links ]
13. Alhalbouni S, Hingorani A, Shiferson A, et al. Iliac-femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg. 2012;26(2):185-189. [ Links ]
14. Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg. 2015;28(1):47-53. [ Links ]
15. Schwein A, Georg Y, Lejay A, et al. Endovascular Treatment for Venous Diseases: Where are the Venous Stents? Methodist DeBakey cardiovascular journal. 2018;14(3):208-213. [ Links ]
16. Dabir D, Feisst A, Thomas D, et al. Physical Properties of Venous Stents: An Experimental Comparison. Cardiovasc Intervent Radiol. 2018;41(6):942-950. [ Links ]
17. Saraf AR, Yadav SP. 2 - Fundamentals of bare-metal stents. In: Wall JG, Podbielska H, WawrzyDska M, eds. Functionalised Cardiovascular Stents: Woodhead Publishing; 2018:27-44. [ Links ]
18. Duda SH, Wiskirchen J, Tepe G, et al. Physical Properties of Endovascular Stents: An Experimental Comparison. Journal of Vascular and Interventional Radiology. 2000;11(5):645-654. [ Links ]
19. Stoeckel D, Bonsignore C, Duda S. A survey of stent designs. Minimally Invasive Therapy & Allied Technologies. 2002;11(4):137-147. [ Links ]
20. Kang CH, Yang SB, Lee WH, et al. Comparison of Open-Cell Stent and Closed-Cell Stent for Treatment of Central Vein Stenosis or Occlusion in Hemodialysis Patients. Iran J Radiol. 2016;13(4):e37994. [ Links ]
21. Marston W. The Critical Need for an Iliofemoral Venous Obstruction Classification System. Endovascular Today. 2017;16(7):48-54. [ Links ]
22. FDA US. Summary of Safety and Effectiveness Data WALLSTENT Venous Endoprosthesis with Unistep Plus Delivery System. Silver Spring - Washington D.C.: U.S. Food and Drug Administration (FDA);2001. P980033. [ Links ]
23. Gagne PJ, Gagne N, Kucher T, Thompson M, Bentley D. Long-term clinical outcomes and technical factors with the Wallstent for treatment of chronic iliofemoral venous obstruction. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2019;7(1):45-55. [ Links ]
24. Gutzeit A, Zollikofer Ch L, Dettling-Pizzolato M, Graf N, Largiader J, Binkert CA. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: long-term results 1987-2009. Cardiovasc Intervent Radiol. 2011;34(3):542-549. [ Links ]
25. O'Sullivan GJ, McCann-Brown JA. Results from VIVO-EU, a Prospective Study of the Zilver Vena Venous Stent in the Treatment of Symptomatic Iliofemoral Outflow Obstruction. Paper presented at: CIRSE 2016; 10 Setembro, 2016; Barcelona. [ Links ]
26. O'Sullivan GJ, Sheehan J Fau - Lohan D, Lohan D Fau - McCann-Brown JA, McCann-Brown JA. Iliofemoral venous stenting extending into the femoral region: initial clinical experience with the purpose-designed Zilver Vena stent. The Journal of Cardiovascular Surgery. 2013;54(2):255-261.
27. de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short-Term Clinical Experience with a Dedicated Venous Nitinol Stent: Initial Results with the Sinus-Venous Stent. Eur J Vasc Endovasc Surg. 2015;50(4):518-526. [ Links ]
28. van Vuuren T, Doganci S, Wittens CHA. Patency rates and clinical outcomes in a cohort of 200 patients treated with a dedicated venous stent. J Vasc Surg Venous Lymphat Disord. 2018;6(3):321-329.
29. Kabnick L. Does Lumen Shape Matter. Paper presented at: American Venous Forum Meeting2018; Tucson,US. [ Links ]
30. Lichtenberg M, Breuckmann F, Stahlhoff WF, Neglen P, Rick G. Placement of closed-cell designed venous stents in a mixed cohort of patients with chronic venous outflow obstructions - short-term safety, patency, and clinical outcomes. Vasa. 2018;47(6):475-481. [ Links ]
31. Razavi M, Marston W, Black S, Bentley D, Neglen P. The initial report on 1-year outcomes of the feasibility study of the VENITI VICI VENOUS STENT in symptomatic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2018;6(2):192-200. [ Links ]
32. Razavi MK. Virtus Clinical Trail. Paper presented at: LINC 2019; Leipiz. [ Links ]
33. Dake M. The BARD® VENOVO" Venous Stent Study - A Prospective, Non-Randomized, Multi-Center, Single-Arm Study of the Treatment of Iliofemoral Occlusive Disease - an Assessment for Effectiveness and Safety (VERNACULAR). U.S. National Library Of Medicine2019. [ Links ]
34. Lichtenberg MKW, de Graaf R, Stahlhoff WF, Ozkapi A, Rassaf T, Breuckmann F. Venovo venous stent in the treatment of non-thrombotic or post-thrombotic iliac vein lesions - short-term results from the Arnsberg venous registry. Vasa. 2019;48(2):175-180. [ Links ]
35. FDA US. Summary of safety and effectiveness data Venovo Venous stent System. Silver Spring- Washington D.C.: U.S. Food and Drug Administration (FDA);2019. P180037. [ Links ]
36. Stuck AK, Kunz S, Baumgartner I, Kucher N. Patency and Clinical Outcomes of a Dedicated, Self-Expanding, Hybrid Oblique Stent Used in the Treatment of Common Iliac Vein Compression. J Endovasc Ther. 2017;24(1):159-166. [ Links ]
37. Medtronic. Abre Venous Self-Expanding Stent System. Endovascular Today Europe. 2018;6(5):101. [ Links ]
38. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. Journal of Vascular Surgery. 1997;26(3):517-538. [ Links ]
39. Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38(1):118-124. [ Links ]
40. te Riele WW, Overtoom TTC, van den Berg JC, van de Pavoordt EDWM, de Vries J-PPM. Endovascular Recanalization of Chronic Long-Segment Occlusions of the Inferior Vena Cava: Midterm Results. Journal of Endovascular Therapy. 2006;13(2):249-253. [ Links ]
41. Raju S, Hollis K, Neglen P. Obstructive lesions of the inferior vena cava: Clinical features and endovenous treatment. Journal of Vascular Surgery. 2006;44(4):820-827. [ Links ]
42. Blattler W, Blattler IK. Relief of obstructive pelvic venous symptoms with endoluminal stenting. Journal of Vascular Surgery. 1999;29(3):484-488. [ Links ]
43. Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflowin chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. Journal of Vascular Surgery. 2007;46(5):979-990.e971. [ Links ]
44. Oguzkurt L, Tercan F, Ozkan U, Gulcan O. Iliac vein compression syndrome: Outcome of endovascular treatment with long-term follow-up. European Journal of Radiology. 2008;68(3):487-492. [ Links ]
45. Black S, Gwozdz A, Karunanithy N, et al. Two Year Outcome After Chronic Iliac Vein Occlusion Recanalisation Using the Vici Venous Stent #xae. European Journal of Vascular and Endovascular Surgery. 2018;56(5):710-718. [ Links ]
46. Lichtenberg M, de Graaf R, Stahlhoff WF, Özkapi A, Simon M, Breuckmann F. Patency rates, safety and clinical results of the sinus-Obliquus venous stent in the treatment of chronic ilio-femoral venous outflow obstruction - data from the Arnsberg venous registry. Vasa. 2018;48(3):270-275. [ Links ]
47. Guillen K, Falvo N, Nakai M, et al. Endovascular stenting for chronic femoro-iliac venous obstructive disease: Clinical efficacy and short-term outcomes. Diagnostic and Interventional Imaging. 2019. [ Links ]
48. Qiu P, Zha B, Xu A, et al. Systematic Review and Meta-Analysis of Iliofemoral Stenting for Post-thrombotic Syndrome. European Journal of Vascular and Endovascular Surgery. 2019;57(3):407-416. [ Links ]
49. Seager MJ, Busuttil A, Dharmarajah B, Davies AH. A Systematic Review of Endovenous Stenting in Chronic Venous Disease Secondary to Iliac Vein Obstruction. European Journal of Vascular and Endovascular Surgery. 2016;51(1):100-120. [ Links ]
50. van Vuuren TM, van Laanen JHH, de Geus M, Nelemans PJ, de Graaf R, Wittens CHA. A randomised controlled trial comparing venous stenting with conservative treatment in patients with deep venous obstruction: research protocol. BMJ Open. 2017;7(9):e017233-e017233.
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: daniela.90.bento@gmail.com (D. Bento).
Recebido a 17 de junho de 2019
Aceite a 23 de agosto de 2019