Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.16 no.3 Lisboa set. 2020
CLINICAL CASE
Late Type 1A endoleak after EVAS: an unique challenge
Endoleak tipo 1A tardio após EVAS: um desafio único
Rita Augusto1,2, Ricardo Gouveia2, Jacinta Campos1,2, Andreia Coelho1,2, Nuno Coelho1,2, Daniel Brandão1,2, Alexandra Canedo1,2
1 Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar de Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal
2 Unidade de Angiologia e Cirurgia Vascular da Faculdade de Medicina da Universidade do Porto, Porto, Portugal
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Endovascular aneurysm sealing (EVAS) using the Nellix system is an alternative method for abdominal aortic aneurysm (AAA) repair. Type 1 endoleaks are not an uncommon complication following conventional endovascular aortic aneurysm repair (EVAR), occurring in up to 10 % of cases. The incidence of these endoleaks following Nellix EVAS was determined to be up to 3,1% in short-term follow-up. Early detection and classification of this issue is crucial to avoid the potential of sac rupture, previously described. As so, we report a successful endovascular treatment of type 1a endoleak, twenty-four months after a Nellix EVAS implantation.
An 82 year-old male underwent a Nellix endovascular repair for a 55 mm infra-renal aortic aneurysm in 2014. Final angiography showed successful aneurysm exclusion with no endoleaks. Regular follow-up using computed tomography angiography (CTA) showed a relatively satisfying good stentgraft positioning, no signs of endoleaks and shrinkage of the aneurysm sac. CTA of 2016 showed a new type 1a endoleak associated wit a significant growth of the aneurysm sac. The authors performed prompt embolization of the endoleak with 0,018” detachable coils and Onyx 34. Final angiography showed patency of the endografts with satisfactory exclusion of the endoleak.
The incidence and significance of type 1 endoleaks following Nellix EVAS was previously studied in literature, with some cases reported and the natural history of untreated type 1 endoleak after EVAS might lead to sac rupture and death. The embolization of the endoleak with coils and Onyx appears to be a safe and effective management choice to achieve technical and clinical success in the treatment of these cases.
Keywords: Abdominal aortic aneurysm; endoleak; Onyx 34; EVAS; Nellix system
RESUMO
Endovascular aneurysm sealing (EVAS) com o auxílio do sistema Nellix é um método alternative para o tratamento de aneurismas da aorta abdominal (AAA). Os endoleaks tipo 1a podem ocorrer em até 10% dos casos de reparação endovascular de AAA (EVAR). A incidência destes endoleaks pode ir até 3,1%, Segundo descrito na literatura. O diagnostico precoce e classificação destes endoleaks são cruciais para evitar casos de rutura do saco aneurismático, previamente descritos. Deste modo, os autores reportam um caso de um endoleak tipo 1a, vinte e quatro meses após realização de EVAS, tratado com sucesso por via endovascular.
Trata-se de um homem de 82 anos de idade, que foi submetido à reparação de um AAA de 55 mm através de EVAS em 2014. A angiografia final demonstrou a exclusão do aneurisma e a ausência de endoleaks. O seguimento pós operatório foi feito como habitual, através da realização de AngioTCs, que demonstravam um correcto posicionamento da prótese, sem sinais de endoleaks e com diminuição progressiva do saco aneurismático. O AngioTC realizado em 2016 demonstrou a presença de novo de um endoleak tipo 1a, associado a um crescimento significativo do saco aneurismático. Face a isto, os autores optaram pela realização de um procedimento endovascular através da embolizaçao do saco com 0,018” detachable coils e Onyx 34. A angiografia final demonstrou a permeabilidade dos componentes da Nellix e a exclusão do endoleak.
A incidência e o significado de um endoleak tipo 1a após EVAS foi previamente estudada na literatura, com alguns casos reportados, e a história natural de um endoleak tipo 1a após EVAS pode levar a rutura do saco aneurismático e consequente morte. A embolização do endoleak com coils e Onyx apresentou-se, neste caso, como uma alternativa eficaz e segura para alcançar o sucesso terapêutico e clínico.
Palavras-chave: Aneurisma da aorta abdominal; endoleak; Onyx 34; EVAS; Nellix
Introduction
Despite improvements in endograft devices, endoleaks of any type are still the most common complications after endovascular abdominal aortic aneurysm repair (EVAR). Type 1a endoleak following conventional EVAR occurs in up to 10 % of cases. These endoleaks usually obligate early re-intervention due to continuing aneurysm sac pressurization and risk of rupture.(1)
Endovascular Aneurysm Sealing (EVAS), using the Nellix system (Endologix, Irvine, CA, USA), is a recent and different method for the treatment of infrarenal abdominal aortic aneurysms (AAAs). This technique is based on two balloon expandable covered 10-mm chromium-cobalt stents, mounted on identical 17F catheter based delivery systems - which provide a flow lumen in parallel from the non-aneurysmal aorta proximally to the iliac artery distally - that are surrounded by endobags that are filled in situ with a soluble polymer to provide both fixation and seal.(2)
The system was designed in an attempt to reduce complications, particularly endoleaks of any kind - due to the ability of the endobags to fill the aneurysm sac, which may impact surveillance strategies and the need for subsequent aortic re-intervention.(2)
The instructions for use (IFU) at its introduction in 2013 included: an infrarenal neck diameter of 18-32 mm, a neck length of 10 mm and neck angle < 60º. After that, the IFU has been redefined to further optimize outcome with a reduction of neck diameter range to a maximum of 28 mm, reduction of the maximum iliac artery diameter to 20 mm and the addition of an AAA/lumen ratio (> 1.4). As the EVAS procedure continued to evolve, a second-generation Nellix device was introduced in 2016 with, amongst other improvements, distal fixation of the endobags to the stents.(2,3 )
The published incidence of endoleak after EVAS is low.(2) Prospective evidence was derived from 2 trials.
The Nellix system Investigational Device Exemption (IDE) pivotal trial, that included 142 patients treated inside the IFU, reported a total endoleak rate at 30 days of 6.3% (type I, 0.7%; type II, 5.6%). At 1 year, the persistent endoleak rate was 3.1% (type I, 0.8%; type II, 2.3%).(4)
The EVAS FORWARD Global Registry, which registered 277 patients both inside and outside the IFU, had an early type Ia endoleak in eight cases. Root cause analysis of the type Ia endoleaks suggested that the majority were due to technical aspects of the procedure: implantation of the device caudal to the optimum sealing zone or insufficient polymer filling of the endobags. The 1-year free survival of type Ia endoleak was 96%.(5)
Although the published incidence of endoleak is low in the short-term - 3,1% -, there are increasing concerns about the durability of the Nellix device. The treatment of a type Ia endoleak after EVAS can be challenging and optimal treatment modalities are yet to be defined, although embolization and proximal extension techniques have been suggested.(2)
In this context, the authors report a successful endovascular treatment of type 1a endoleak twenty-four months after a Nellix EVAS implantation.
Case report
An 82 year-old male with a prior medical history of hypertension, dyslipidemia and smoking, underwent a Nellix endovascular repair for a 55 mm infra-renal aortic aneurysm in 2014.
On preoperative contrast computed tomography (CT) imaging, the AAA had a blood lumen diameter of 42 mm. The proximal aortic neck had a maximum diameter of 24 mm, a length of 20 mm from the lower renal artery. The maximum diameters of the iliac arteries were 13 mm on the right an 18 mm on the left. EVAS was performed within the company’s instructions for use of Nellix. Percutaneous bilateral transfemoral access was obtained using percutaneous approach.
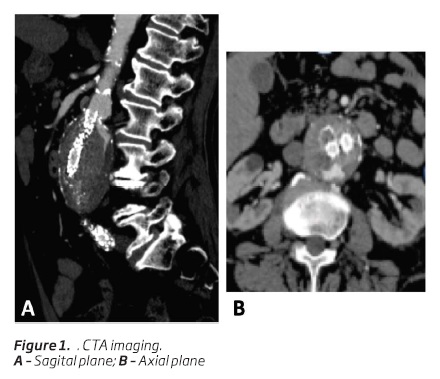
Final angiography showed successful aneurysm exclusion with no endoleaks. Regular follow-up using computed tomography angiography (CTA) showed a relatively satisfying good stentgraft positioning, no signs of endoleaks and a shrinkage up to 52 mm of the aneurysm sac - CTA performed at 1, 6, 12 and 24 months. CTA at 24 months follow-up showed a new type 1a endoleak associated wit a significant growth of the aneurysm sac up to 58 mm (Figure 1). As so, the authors decided to perform prompt embolization of the referred endoleak.
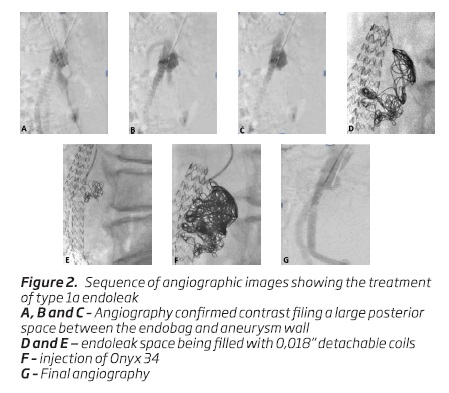
Ultrasonography guided left brachial artery access was obtained, a 4 Fr 70 cm sheath was placed and an angiogram confirmed contrast filing a large posterior space between the endobag and aneurysm wall.
After catheterizing the endoleak space with a 4 Fr MP catheter, a 2,7 Fr microcatheter was placed and the endoleak space was filled with 0,018” detachable coils. After that, Onyx 34 (Covidien, Irvine, California, USA) was slowly injected into the interstices between the coils to provide complete occlusion of the endoleak cavity (Figure 2). The authors prefer to use only detachable retrievable microcoils because of the potential for coil misplacement and migration out of the endoleak cavity due to a whirlpool phenomenon.
Final angiography showed patency of the endografts with satisfactory exclusion of the endoleak. There was no clinical evidence of distal embolization foci and the patient was discharged uneventfully.
CTA and US performed at 1 and 6 months after procedure showed no endoleaks and a shrinkage of the aneurysm sac up to 56 mm.
Unfortunately, the patient died 15 months after the re-intervention, due to a respiratory infection with sepsis.
Discussion
Initial efficacy data on the EVAS technique was encouraging, but the knowledge of its potential complications and their respective managements are limited. Reported adverse events include migration and proximal type 1 endoleak, type 2 endoleaks, graft stenosis and occlusion.
After the commercial release of Nellix in 2013, in the largest cohort of patients to date, Böckler and colleagues(6) reported their experience in 171 cases, performed at multiple European centers and one in New Zealand. Technical success (98.8%) was achieved in all but two patients. They found no intra-operative type 1a endoleaks, but five type 1a endoleaks were detected on follow-up, three after one month and two at six months. One of these resolved spontaneously, two were embolized and two were observed. There was no difference in the aortic neck length in cases with or without type 1a endoleaks (22+/-12 mm vs 28+/-15 mm; p=0.39). Four of five type 1a endoleaks were seen in the 116 cases within the manufacturer’s IFU and one within the 55 cases outside the IFU. There were four limb occlusions (2.3%). Aneurysm-related re-interventions were performed in nine patients (5.2%) and there were no aneurysm ruptures or surgical conversions.
The data available currently presents the short-term follow-up results and the endoleaks observed may be in part related to technical factors during the deployment, resulting in lower than intended positioning of the proximal graft and insufficient coverage of the proximal neck.(7)
According to EVAS Type IA Endoleak Study Group(2), post-EVAS type Ia endoleaks after EVAS were categorized as follows: a type Is1 endoleak was defined as the appearance of contrast between the endobag and the wall of the proximal neck but not reaching the aneurysm sac. This type would not be classified as endoleak within the accepted definitions for EVAR. Type Is2 endoleaks were defined as those where there was contrast between the endobag and aneurysmal wall or thrombus inside the aneurysm sac - as it was shown in this case; a type Is3 endoleak was defined as showing contrast or newly formed thrombus between the endobags inside the aneurysm sac and a type Is4 endoleak was defined as the presence of sac pressurisation without proof of endoleak or with the presence of secondary signs.
There are no data on the natural history of untreated endoleak following EVAS but it seems intuitive to assume that there is a similar potential for sac enlargement and ultimately rupture. In fact, there has been a case report in the literature of a delayed recurrent type 1a endoleak associated with increased aortic sac size and rupture.(8)
Sealing of proximal type 1a endoleaks after an EVAR may be treated by placement of a proximal cuff extension (with or without chimney grafts to the renal and/or visceral arteries), branched/fenestrated EVAR, EndoAnchors, endovascular embolization, or open surgery. Although, these approaches are not possible with the Nellix system for obvious reasons. An alternative approach is transcatheter embolisation with Onyx, which is well established for type 2 endoleaks.(9)
The literature on the management of type 1a endoleak following EVAS is very limited.
Partial or total endograft explanation combined with conventional open repair remains an option for patients with a persistent type Ia endoleak that is not amenable to endovascular therapy (including branched or fenestrated solutions).(3)
Ameli-Renani et al, published their experience on embolization of 7 type 1a endoleaks after EVAS - using coils and Onyx in six cases and Onyx only in one case. They obtained a 100% technical success no recurrent endoleaks during the follow-up.(10) One of the concerns related to this treatment is the possibility of Onyx reflux during the injection, which may compromise the patency of the endografts or lead to distal embolisation. For this reason, some authors prefer to initially use detachable coils to form a scaffold in the endoleak cavity before completing the procedure with Onyx.(10) A disadvantage of Onyx and coils embolisation is the streak artifact present on follow-up CT imaging which can obscure a recurrence of the endoleak.(7)
Distal migration - the commonest cause of late endoleaks - can be treated with proximal Nellix-in-Nellix extension. This technique, however, has limitations, as the endobags of the Nellix extensions should protrude at least 2 to 3 cm above the primary stent in order to provide good wall apposition. Given this minimum sealing length requirement, parallel grafts for the renal arteries are often required.(3) In patients with caudal migration who still have an adequate length of proximal neck seal, reinforcement of the Nellix stents with rigid, balloon-expandable covered stents might also be of value. In this case, after the image study, we can conclude that the cause of the endoleak was the development of aneurismal aortic disease in the proximal neck.
Long-term durability of all these re-interventions needs to be confirmed.
According to the literature, Nellix EVAS has also been successfully used to repair failed EVAR, ruptured abdominal aortic aneurysm and iliac artery aneurysms.(11-13)
The new revised IFU and latest-generation Nellix promise good results, even though patient applicability is significantly reduced. This needs clinical validation and the continuation of the Nellix EVAS FORWARD clinical trial provides an opportunity for that.(3)
Conclusion
The incidence and significance of type 1 endoleaks following Nellix EVAS is unknown with few cases reported and there is no data on the natural history of untreated type 1 endoleak after EVAS. Unlike conventional EVAR, the alternatives to treat a type 1a endoleak after EVAS are clearly reduced, transforming it into a difficult therapeutic challenge. Meanwhile, the embolisation of the endoleak with coils and Onyx appears to be a safe and effective management choice to achieve technical and clinical success in the treatment of these cases.
REFERENCES
1. Green N, Sidloff DA, Stather PW, et al. Endoleak after endovascular aneurysm repair: 189 current status. Rev Vasc Med. 2014;2:43-7. [ Links ]
2. Van den Ham LH, Holden A, Savlovskis J, et al; EVAS Type IA Endoleak Study Group. Occurrence and classification of proximal type I endoleaks after endovascular aneurysm sealing using the Nellix device. Eur J Vasc Endovasc Surg. 2017;54:729-736. [ Links ]
3. Reijnen MM, Holden A. Status of endovascular aneurysm sealing after 5 years of commercial use. Journal of Endovascular Therapy. 2018;25(2):201-206. [ Links ]
4. Carpenter JP. Nellix System for endovascular aneurysm sealing: effect of IFU refinements from the EVAS FORWARD IDE. Paper presented at: The 2017 Vascular Annual Meeting of the Society for Vascular Surgery; May 31-June 3, 2017; San Diego, CA. [ Links ]
5. Thompson MM, Heyligers JM, Hayes PD, et al, for the EVAS FORWARD Global Registry Investigators. Endovascular aneurysm sealing: early and midterm results from the EVAS FORWARD Global Registry. J Endovasc Ther. 2016;23:685692.
6. Böckler D, Holden A, Thompson M, et al. Multicenter Nellix EndoVascular Aneurysm Sealing system experience in aneurysm sac sealing. J Vasc Surg. 2015;62:290-298. [ Links ]
7. Ameli-Renani S, Morgan RA. Secondary interventions after endovascular aneurysm sac sealing: endoleak embolization and limb-related interventions. Seminars in Vascular Surgery. 2016 Mar;29(1-2):61-7. [ Links ]
8. Pua U, Quek LHH, Tan GWL. Delayed Recurrence of Type 1A Endoleak with Aortic Rupture and Hemorrhage After Endovascular Aneurysm Sealing (EVAS). CardioVascular and Interventional Radiology. 2016 Jul;39(7):1061-5. [ Links ]
9. Ameli-Renani S, Das R, Weller A, et al. Embolisation of a proximal type I endoleak postnellix aortic aneurysm repair complicated by reflux of onyx into the nellix endograft limb. Cardiovasc Intervent Radiol 2015;38(3):747-751. [ Links ]
10. Ameli-Renani S, Morgan RA. Transcatheter embolisation of proximal type I endoleaks following endovascular aneurysm sealing (EVAS) using the Nellix device: technique and outcomes. Cardiovasc Intervent Radiol. 2015;38:1137-1142. [ Links ]
11. Böckler D, Reijnen MM, Krievins D, et al. Use of the Nellix EVAS system to treat post-EVAR complications and to treat challenging infrarenal necks. J Cardiovasc Surg (Torino). 2014;55:601-612. [ Links ]
12. Reijnen MM, de Bruin JL, Mathijssen EG, et al. Global experience with the Nellix Endosystem for ruptured and symptomatic abdominal aortic aneurysms. J Endovasc Ther. 2016;23:21-28. [ Links ]
13. Krievins DK, Savlovskis J, Holden AH, et al. Preservation of hypogastric flow and control of iliac aneurysm size in the treatment of aortoiliac aneurysms using the Nellix EndoVascular Aneurysm Sealing endograft. J Vasc Surg. 2016;64:1262-1269. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: rita.augusto1988@gmail.com (R. Augusto).
Recebido a 10 de maio de 2020. Aceite a 30 de setembro de 2020.