Introduction/objectives
Aorto-iliac occlusive disease (AIOD) usually develops at the aortic bifurcation and may be associated to proximal and distal progression, leading to complete aortic thrombosis in 3-15% of the cases.(1-6)
Due to the development of collateral circulation, symptoms may be mild and well tolerated and patients presenting with intermittent claudication do not usually progress to chronic limb threatening ischemia.
However, the thrombus may extend proximally to the renal arteries and visceral aorta, leading to deterioration of the renal function and developing or worsening of severe arterial hypertension.
We report the uncommon case of a patient with aortic thrombosis associated to occlusion of the left renal artery.
Case report
The following case is reported after informed consent was given by the patient.
A 56-year-old man, heavy smoker and with no other risk factors for atherosclerosis, presented in 2018 at the outpatient clinic with disabling intermittent proximal claudication, grade I and category 3 in the Rutherford classification, and absent femoral pulses. There were no complaints of sexual disfunction. Aspirin, sinvastatin and cilostazol were prescribed, regular physical activity and smoking cessation were encouraged.
The color-flow duplex scan showed the presence of severe aorto-iliac occlusive disease, direct signs of infra-renal aortic occlusion and monophasic wave patterns in both femoral arteries. There was no evidence of femoro-popliteal or tibio-peroneal occlusive disease.
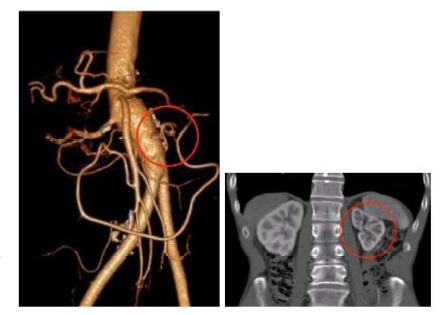
A CT-angio was requested and revealed juxtarenal aortic thrombosis without the involvement of the renal arteries (Figure 1), extending through the common and proximal external iliac arteries with heavily calcified walls. The femoro-popliteal and tibio-peroneal sectors did not present significant occlusive disease.
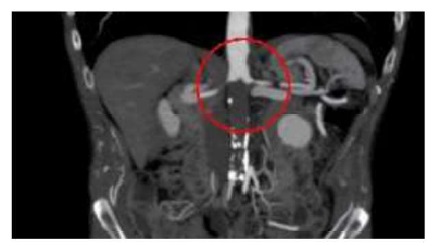
Figure 1 Coronal view of the pararenal aorta showing juxtarenal aortic thrombosis, with patent renal arteries (2018).
The patient was proposed for an aortic thrombectomy and aorto-bifemoral interposition graft and was included in a surgical waiting list with low priority due to the chronic claudication status.
During the waiting period the symptoms remained stable but with little improvement despite the given medication.
In 2020, the patient was summoned for surgery.
The general medical assessment was normal, with no cardiac, pulmonary, renal or metabolic impairment. A new CT-angio was performed and showed the proximal extension of the thrombus (Figure 2A), with occlusion of the left renal artery (LRA) and delayed perfusion of the left kidney, which measured 7cm (bipolar diameter) (Figure 2B). There was also a layer of thrombus in the lateral aspect of the aorta extending to the celiac trunk level (Figure 2A).
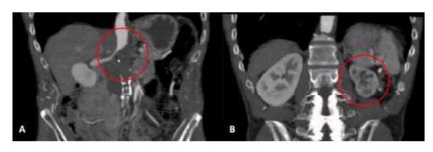
Figure 2 A. Coronal view of the pararenal aorta showing juxtarenal aortic thrombosis involving the LRA (2020). B. Coronal view of the left kidney showing the presence of contrast perfusion despite main artery occlusion.
With these new findings, the surgical strategy changed, and the patient was proposed for a medial visceral rotation approach.
The access to the whole aorta, from the supraceliac level to the bifurcation, was obtained through a paramedian Risberg incision with medial visceral rotation followed by tunneling to the femoral arteries.
After systemic heparinization (2000 units), supraceliac aortic clamping was performed followed by longitudinal aortotomy which allowed the visualization of the ostia of the celiac trunk (CT), superior mesenteric artery (SMA) and right renal artery (RRA). After proper thromboendarterectomy of the para-visceral aorta, the RRA was cannulated and perfused with cold Ringer’s lactate solution. The proximal end-to-end anastomosis was then performed and including the CT, SMA and RRA ostia.
The supraceliac clamping time was 23 minutes and the patient resumed diuresis after 4 minutes.
We also decided to revascularize the left kidney taking into account that the distal left renal artery was patent, there was late kidney perfusion on the CT-angio and the bipolar diameter was 7cm. This artery was managed by eversion endarterectomy and implantation on the bifurcated graft (Dacron 16x8mm, Jotec® , Figure 3).
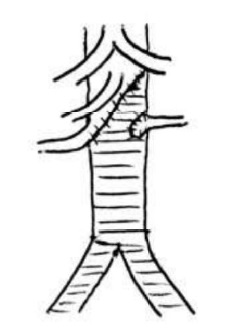
Figure 3 Schematic view of the proximal anastomosis involving the CT, SMA and RRA and reimplantation of the LRA in the graft
Distally, the graft was anastomosed to the common femoral arteries.
The surgical time was 270 minutes, blood loss was estimated around 1000mL and two red blood cell packs were transfused. Additionally, 4500mL of polyelectrolytic solution were infused with a total intraoperative diuresis of 1600mL.
The post-operative period was uneventful, the lower limb pulses recovered normally, and the patient was discharged at day 5 post-intervention.
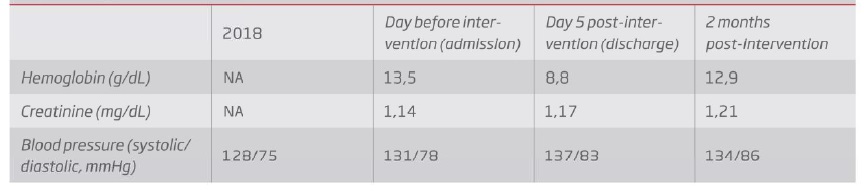
After two months, the patient presented no claudication symptoms, maintained femoral pulses, normal blood pressure values and no significant changes in serum creatinine levels (Table 1).
Table 1 Evolution of hemoglobin, serum creatinine and blood pressure values through time, before LRA occlusion (2018), before and after intervention. NA - Non available
The control CT-angio showed the integrity of all anastomoses and the patency of the four visceral vessels (Figure 4A). There was also improvement in contrast captation by the left kidney (Figure 4B).
Discussion/conclusions
We presented a case of juxtarenal aortic thrombosis that progressed to the supra-renal level and led to renal occlusion in a 2-year period. This uncommon complication was assessed by the study of Starret in 1974(2) which found that it may occur in one third of the cases throughout a period of 5 to 10 years. However, more recent studies did not confirm these findings and found this complication to be more uncommon than previously thought. The involvement of the renal arteries seems to have an estimated prevalence of 3-15%.(3-6)
Due to the risk of loss of renal mass and even intestinal ischemia, the treatment of patients with juxtarenal aortic thrombosis should be considered.
Another consequence of thrombus progression to the celiac level is the need to change the surgical approach to a more difficult procedure with supraceliac clamping and associated to higher potential of complications.
In this case, we decided to revascularize the lower limbs due to the disabling symptoms and we chose a conventional approach due to the young age and good physiological reserve of the patient.
Regarding the decision for LRA revascularization, we should keep in mind that in some cases of occlusion of the main renal artery the pericapsular circulation may preserve the kidney through the adrenal and lumbar arteries(7,) allowing the revascularization of a viable kidney.(8,9) Our criteria to proceed to revascularization of an occluded renal artery are the distal patency of the artery, the renal bipolar diameter >7cm(8,9) and the late contrast perfusion in the CT-angio.
While there is an ample discussion regarding medical treatment or renal revascularization in atherosclerotic renal artery disease, with several randomized controlled trials reporting no significant advantage of stenting(10-12), there are specific clinical settings where revascularization, endovascular or surgical, are clearly indicated and that were not considered in the above trials. Studies from the 1990’s showed the long-term functional benefit of revascularization in selected patients with renal occlusion(13), also in patients submitted to thoracoabdominal or suprarenal aneurysm repair.(14-17)
When there is a need to clamp the supraceliac aorta, the use of adjuvant techniques to protect the kidneys is beneficial. Solutions like cold Ringer’s lactate(17-19) and more recently custodiol(20) are established strategies to decrease the risk of postoperative renal failure, streadily available in our center with a simple perfusion technique (IV bag, tube and Pruitt irrigation catheter).
In conclusion, the progression of juxtarenal aortic thrombosis to the supra-renal level is uncommon, associated to loss of renal mass and may implicate more challenging surgical procedures. When a surgical indication is considered, these patients, should not wait for long periods of time.