Introduction
Peripheral arterial disease (PAD) affects more than 200 million people worldwide and its prevalence is increasing, especially in low-income countries1. The best-known risk factors include age, active smoking, diabetes mellitus (DM), arterial hypertension, dyslipidemia, and chronic kidney disease2. With the decrease in the prevalence of smoking habit in western countries, diabetes mellitus gains relevance as one of its major risk factors nowadays. It is known that the longer the duration of diabetes the greater the risk of developing peripheral arterial disease and thus patients with type 1 diabetes mellitus are particularly prone to this complication at an early age3.
Pancreas-kidney transplantation has changed the course of type 1 DM patients with end-stage renal disease (ESRD) management. It provides a physiological mean of achieving normoglycemia with the advantage of keeping the ESRD patient free of dialysis4. A successful pancreas transplantation, by allowing the endocrine functions of the pancreas to be restored and by optimizing metabolic control, will intuitively slow the progression of diabetic micro and macrovascular complications. In fact, it has been reported that long-term normoglycemia following successful simultaneous pancreas kidney transplant (SPKT) significantly withstands improvement of the microangiopathic diabetic injuries, and that some of the already established injuries even have the potential to be reversed5,6. In contrast, data on the evolution of macroangiopathic diabetic injuries, as PAD, after SPKT is far from clear.
PAD is an independent predictor of cardiovascular events, affecting both the quality and expectancy of life. Early diagnosis and management of this disease is crucial to delay or avoid amputation and improve prognosis7. However, in patients undergoing SRKT, metabolic imbalance and renal disease represent the major burden for the development of the disease and the influence on other classical risk factors is still not well understood. Also, the characterization of the influence of PAD on overall survival is unknown in this group of patients.
Methods
Study design and population
This research is a retrospective observational study, based on the electronic clinical records of 229 patients with type I Diabetes Mellitus and End Stage Renal Disease who underwent SPKT at Centro Hospitalar Universitário do Porto between May of 2000 and December of 2019. At our institution, SPKT indications include every fit for surgery patient with type I Diabetes Mellitus and End Stage Renal Disease requiring kidney transplant.
From the 229 patients, 13 patients were excluded from the study - 6 died at the time of transplant and 7 were lost in follow-up.
Risk factors and comorbidities
We conducted an extensive review of all patients' clinical records, and data describing their demographic and clinical features were collected, including sex, age, age at transplant, Body Mass Index (BMI), years of diabetes prior to transplant, months of dialysis prior to transplant, smoking, antihypertensive drugs intake, statins intake, cerebrovascular disease, myocardial ischemia, retinopathy, cholesterol levels (TC, LDL-C, HDL-C), triglycerides, HbA1c levels, creatinine, cystatin C, CRP, albumin and levels of neutrophils, lymphocytes, and platelets. To define the presence of dyslipidemia, we used HDL <40 mg/ dl in men and <50 mg/ dl in women, LDL > 120 mg/ dL, total cholesterol > 200 mg / dl and triglycerides ≥ 150 mg / dl, as criteria8. We also registered all follow-up periods, grafts’ viabilities and grafts’ survival time.
Peripheral arterial disease assessment
Due to the retrospective nature of our research and considering that the ankle brachial index was not systematically evaluated in all patients undergoing SRKT we only considered the presence of peripheral arterial disease in patients who were symptomatic: intermittent claudication or chronic limb threatening ischemia (CLTI) - rest pain and ulceration.
A detailed review of the presentation of patients with manifestations of PAD was performed according to the Rutherford scale2. The revascularization procedures were classified as endovascular, surgical or hybrid. The anatomical classification of the lesions was performed similarly to Dihem et al: aortoiliac (common, external and internal iliac arteries), femoropopliteal (common, superficial and deep femoral arteries, popliteal artery) and infrageniculate or distal (tibiofibular trunk, anterior and posterior tibial arteries, fibular artery) disease9.
Considering the high frequency of aortoiliac disease found in patients with peripheral arterial disease, in disagreement with the known pathophysiology of diabetes mellitus typically associated with a more distal disease, a sub-analysis of these patients was carried out, investigating the technical aspects of transplantation surgery and as well as the detailed anatomical characterization of the disease.
Statistical analysis
The statistical analysis was performed using the IBM SPSS Statistics version 27.0.
The research results are presented in the form of mean ± standard deviation or median (interquartile range) for quantitative variables if they were normally or non-normally distributed, respectively. Qualitative variables are expressed as absolute numbers and percentages.
The study groups stratified as patients with PAD and patients without PAD were compared on clinical characteristics using independent t-test for continuous normally distributed variables,
Mann-Whitney test for continuous non-normally distributed variables and chi-square test for categorical variables.
The odds ratios (OR) for prevalent PAD associated with the cross-classification of LDL cholesterol, myocardial ischemia, HbA1c 6 months and 3 years after transplant and Cystatin C 5 years after transplant were calculated using logistic regression models.
A Kaplan-Meier survival analysis was performed to study the influence of PAD on the patients’ survival, as well as on the of the pancreas and kidney grafts survival rates.
For the statistical significance, a p-value < 0.05 was established. A confidence interval of 95% was used.
Results
Characteristics
Our study was conducted on a group of 216 patients - 111 (51.4%) men and 105 (48.6%) women - with mean age of 46.01 ± 0.48 years and mean age at transplant of 35.54 ± 6.02 years. Mean follow-up time was 9 years.
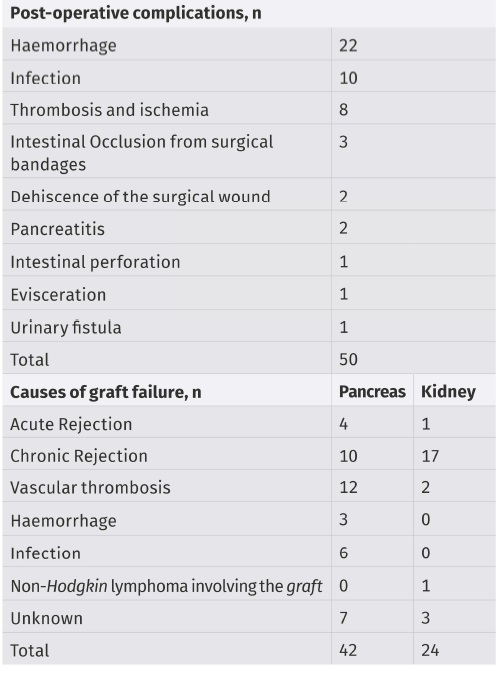
In 50 patients (23.15%) there was a need for reintervention in the same hospitalization because of different post-operative complications. During the follow-up period, the pancreatic graft remained functional in 174 (80.56%) patients, six patients with failed pancreas graft received a second pancreas transplant and it remained functional in only one patient. Regarding the kidney transplantation, the kidney graft remained functional in 192 (88.89%) patients Five of the patients with lost grafts were then re-transplanted and the rest started a hemodialysis program. Two of the retransplanted patients maintained a functioning graft whereas the 3 others lost the graft and begun hemodialysis. The post-operative complications as well as the causes of graft loss are detailed in Table I.
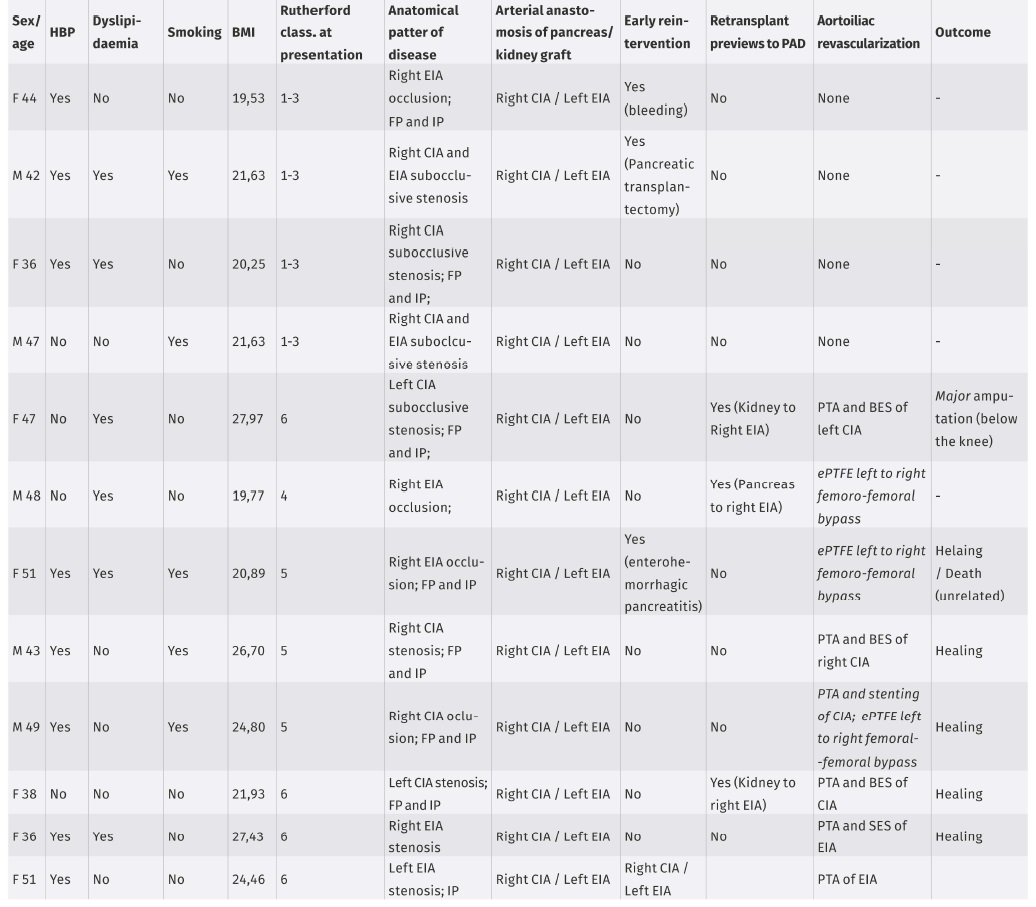
The PAD characteristics of the 32 symptomatic patients are described in Table II. Eight patients (25%) presented with intermittent claudication and no revascularization procedures were performed. CLTI was targeted in 24 patients (75%) and 23 of these patients had undergone one or more surgical and endovascular revascularization procedures. In revascularized patients the rate of major amputation was 21% (n=6). One patient presented a small ulcer on the foot with Doppler ultrasound documenting hemodynamically significant occlusive disease of the infrapopliteal sector, however, he presented a favorable evolution of the lesion without the need for an invasive procedure for revascularization.
Aortoiliac arterial disease sub analysis
Twelve of the symptomatic patients had disease of the aortoiliac sector documented in diagnostic tests (table III). Only in one patient the disease was limited to this anatomical sector, affecting multiple lower limb territories in all others. Four patients (33%) presented with complaints of intermittent claudication and 8 patients (67%) with chronic limb-threatening ischemia. In 9 patients (75%) the hemodynamically significant arterial disease was in the right iliac axis and in 3 patients (25%) in the left iliac axis. Six patients (50%) had hemodynamically significant lesion (stenosis or occlusion) in the transplant artery anastomosis: 5 patients with lesion in the right common iliac artery where the pancreatic transplant anastomosis was performed and 1 patient with lesion in the left external iliac artery where the renal transplant anastomosis was performed.
Analysis of predictive factors
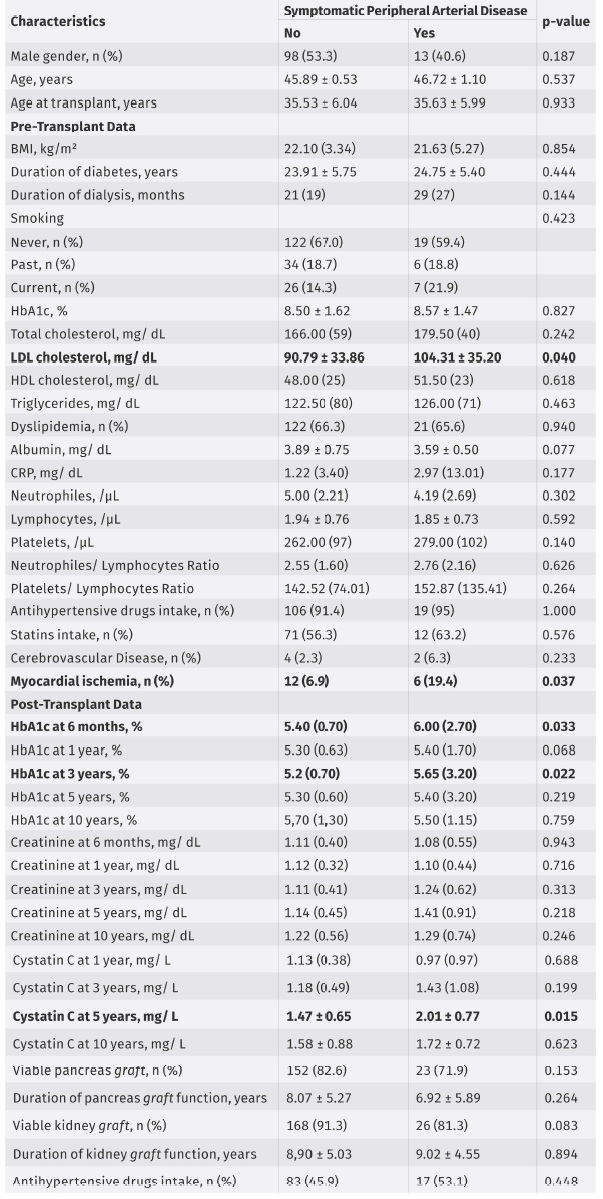
Patient age, age at transplant, sex and BMI were not significantly different between the two groups.
The duration of diabetes mellitus prior to transplantation was mean 23.91 ± 5.75 years in the non- PAD group and mean 24.75 ± 5.40 years in PAD patients, with a non-significant difference. In non- PAD, all patients except 8 underwent hemodialysis as a replacement therapy for renal function before transplant with a median duration of hemodialysis of 21 (19) months. Whilst in PAD, only 1 patient did not undergo hemodialysis, with a median duration of hemodialysis 29 (27) months in this group. The duration of hemodialysis prior to transplant did not show any statistically significant difference between the groups.
Regarding smoking, we found no statistically significant difference between the two groups; although former and current smoking were slightly higher in the PAD patients. No association was found between antihypertensive therapy and statins intake and the prevalence of PAD.
There was an association found between PAD and myocardial ischemia (p = 0.037) with a higher risk of developing PAD of 3.220-fold among patients with myocardial ischemia. No association between PAD and cerebrovascular disease was found.
Patients with PAD were characterized by higher pre-transplant LDL-C levels (p = 0.040) and higher LDL-C levels were associated with a 1.011-fold higher risk of developing PAD. TC, HDL-C and triglycerides did not reveal any significant difference between the two groups. Regarding inflammation associated markers, no significant difference was found in CRP, NLR and PLR between groups.
HbA1c was significantly higher at 6 months (p = 0.033) and at 3 years after transplant (p = 0.022) in PAD patients, being associated with a 1.512- and a 1.334-fold higher risk of developing PAD, respectively. Besides that, at 1 and 5 years after transplant it was also higher in PAD patients, tending towards statistical significance 1 year after transplant (p = 0.068). Cystatin C was significantly higher in PAD patients 5 years after transplant (p = 0.015) and associated with a 2.405 higher risk of developing PAD. At 3- and 10-years’ time although Cystatin C was also higher in PAD patients, the difference was not statistically significant. Regarding creatinine levels no significant difference was found.
In patients with no PAD symptoms, the mean duration of the pancreatic graft was 8.07 ± 5.27 years and of the kidney graft was 8.90 ± 5.03 years while in PAD patients it was 6.92 ± 5.89 years and 9.02 ± 4.55 years, respectively; no statistically significant difference was found.
Analysis of survival
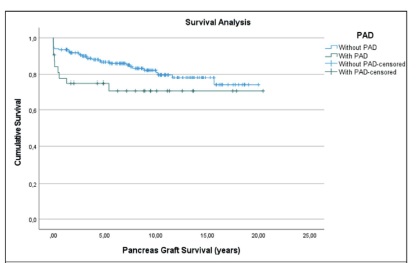
Regarding the influence of PAD on pancreas and kidney grafts survival, using Kaplan Meieranalysis (Figures 1 and 2), even though no statistically significant difference was found, we found an interesting trend towards lower graft survival in PAD patients, especially regarding kidney graft survival Figure 2.
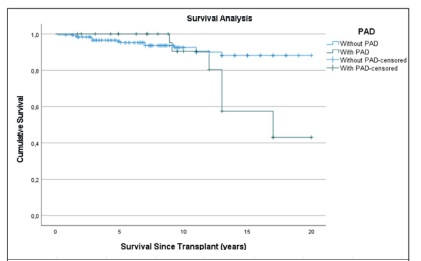
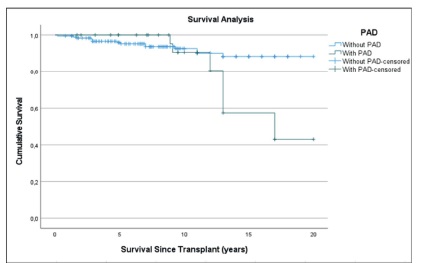
Through the Kaplan-Meier survival analysis performed to evaluate the effect of PAD on patients survival, although the difference between the two groups was not statistically significant, PAD was clearly associated with lower survival time. Analyzing Figure 3,it is evident that from 10 years’ time after transplant there is a much steep decline in survival among PAD patients compared to non- PAD patients.
Discussion
In this study, over the mean 9 years of follow up, the percentage of patients who developed symptomatic PAD was 14.81% which is an expressive value considering that the average age of our population was 46 years. In general population at age 45-49 years the prevalence of PAD is estimated to be 5.28% in women and 5.41% in men, mostly asymptomatic10.
The duration of diabetes and hemodialysis prior to transplant did not show a significant role in the development of PAD in this population. Regarding smoking, one of most powerful predictor of large vessels PAD progression11, no difference between the groups was found in our population.
The presence of myocardial ischemia associated with a 3.220-fold higher risk of developing PAD, which is in agreement with the systemic pathophysiology of atherosclerosis in multiple territories12. In contrast, in our study, we did not find any clear association between cerebrovascular disease and PAD.
The significance of high LDL-C as a major atherosclerotic risk factor and the importance of intensive LDL-C lowering in patients who are at high risk of atherosclerotic disease is well established13. Our results on LDL-C are is in agreement with these findings and proves that in SPKT population, metabolic control is crucial.
Also, in accordance with our findings regarding HbA1c, in the prospective Heinz Nixdorf Recall Study, diabetes with poor glycemic control (indicated by HbA1c≥7.0%) was strongly associated with incident PAD over a 5-year and 10-year follow-up14. It was also showed that a higher mean of HbA1c and a higher variability indicate an increased risk for PAD15.
Several studies suggest that clinical markers associated with inflammation, such as CRP, lymphocytes and platelets, can be associated with the development of PAD16,17. However, in our study, none of those correlations were found.
Cystatin C is independently associated with incident PAD during long-term follow-up18. In our study, although there was a correlation between cystatin C elevation and the development of PAD, the same was not observed for creatinine, which suggests that this marker can have an impact on the development of PAD regardless of kidney dysfunction. Other studies also reported that elevated cystatin C concentration was found to be associated with future PAD procedure (bypass surgery, angioplasty, or amputation) among persons who did not have PAD at baseline19.
Although some studies found association between the viability of the pancreas and kidney grafts and the development of PAD20, in our study we did not find that association, which is possibly justified by the small number of lost grafts. However, the kidney graft survival seems to be lower in PAD patients which is shown in the analysis of the Kaplan-Meier curve in Figure 2. Also regarding the Kaplan-Meier survival analysis - Figure 3 - it is evident that from 10 years’ time after transplant there is a much steep decline in survival among PAD patients compared to non-PAD patients which comes in agreement with several studies that have already showed that PAD is a risk factor for all-cause mortality7,21.
Another interesting finding of our study was the relative high prevalence of aortoiliac arterial disease (38% of all symptomatic patients) that would not be expected as DM is described in several studies by causing more distal atherosclerosis located mainly below the knee and because DM has been reported as the most obvious risk factor for atherosclerotic involvement of infragenicular arteries9. Possible explanations for this aortoiliac segment disease is the vascular clamp injury caused in the vessel at the time of transplant with subsequent intimal hyperplasia22 and the hemodynamic impact of anastomosis performed at time of transplant. However, data in the literature are scarce regarding the development of iliac stenosis in long-term transplant patients.
This study has several limitations which may have compromised our results. Two limitations were that our study was a single center study, and it was retrospective.
Conclusion
Our study has come to agreement that the lack of metabolic control, namely the elevated levels of LDL cholesterol and HbA1c seem to contribute to the development of the PAD disease. Elevated levels of cystatin C were also associated with PAD and this can be an independent marker of progression of disease. Additionally, this study demonstrated that patients with myocardial ischemia were at higher risk of developing PAD. Besides the pathophysiology associated with diabetes mellitus and chronic kidney disease, our study suggests that vascular clamp injury may also play a role in the development of peripheral arterial disease in these patients. Also, the development of DAP could be associated with lower kidney graft and patient survivals rates, namely 10 to 15 years after the transplant surgery.