Introduction
Endovascular aneurysm repair (EVAR) offers significant advantages on aneurysm treatment and is nowadays the most common operation for infrarenal abdominal aortic aneurysms. Despite this, there are concerns about the long-term durability of these procedures, requiring image follow-up and associated with increased secondary intervention rates.
Delayed rupture risk after EVAR has been described as 1% per patient per year.1,2,3 Ultimately EVAR failure results in complex surgical approaches, sometimes requiring graft explantation which remains a major challenge and one associated with a high morbidity and mortality. The purpose of this study is to review our contemporary institutional experience with EVAR explantation.
Methods
The authors declare that they have followed the protocols of their centre on the publication of retrospective patient data and comply with the Helsinki declaration on research ethics.
A retrospective analysis of an institutional administrative database was performed to identify patients who were subject of graft explantation following standard infra-renal EVAR between 2011 and 2021. Follow-up was extracted from patient charts.
The primary endpoint was perioperative mortality (30-days or in-hospital mortality). Demographics, major comorbidities (hypertension, diabetes, ischemic heart disease, pulmonary disease, renal disease and cerebrovascular disease), indication for EVAR, type of graft used, compliance with Instructions for use (IFUs) (infrarenal neck angulation, neck length, neck calcification, neck thrombus, iliac tortuosity and proximal and distal diameters) indications for explantation (rupture, endoleak with sac enlargement, infection, aorto-enteric fistula), procedure details (transperitoneal vs. retroperitoneal approach, clamp position, type of reconstruction, partial or complete explantation) and outcomes (complications, intra-operative death, death in the first month, return to the operating room) were evaluated. A descriptive analysis of the data was performed.
Results
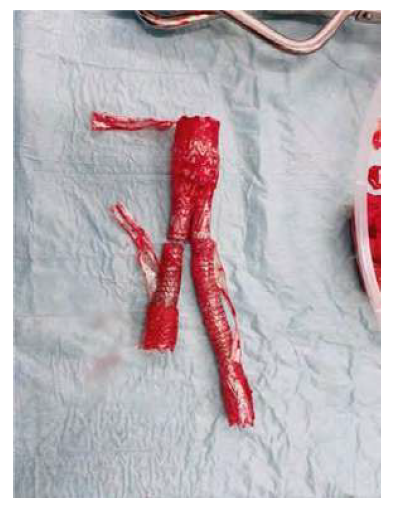
We identified 13 patients submitted to EVAR explantation (figure 1), over a 10-year period, between 2011 and 2021, two of which were referrals from other vascular centers. During this period, there were 617 standard primary EVAR procedures performed in our institution for infrarenal aortic aneurysms. This accounts for an explantation rate of less than 2% over 10 years.
All patients were male and median age at explantation was 72 years (range 47-81). 85% of patients (eleven patients) had hypertension, 23% (three patients) were either active smokers or had a history of pulmonary disease (chronic obstructive or restrictive pulmonary disease), 23% (three patients) had a previous acute coronary event, 23% (three patients) had some degree of renal disfunction (Glomerular Filtration Rate below 60 ml/min/1.73m2), 15% (two patients) had diabetes, 8% (one patient) had a previous cerebrovascular event.
The primary indication for EVAR (Table 1) was ruptured infrarenal aortic aneurysm in 54% (seven) of patients. The primary EVAR procedure took place 29 months (range 0-72) before explantation. Explanted endografts included eight Endurant (Medtronic), two Excluder (W. L. Gore & Associates), one Talent (Medtronic), one Nellix (Endologix) and one Zenith (Cook Medical). Eleven of the explanted endografts were aorto-bi-iliac, and two were aorto-uni-iliac (both with adjunct simultaneous femoro-femoral bypass).
The majority of explantation operations were emergent (6/13, three due to unstable aorto-enteric fistula (AEF), three due to rupture) or urgent (4/13, two stable AEF, two graft infections). In 3 cases, explantation was elective (two type Ia endoleaks and one type II endoleak with sac expansion).
Table 1 Indications for EVAR, explantation and surgery employed.
| Indication for EVAR | Months to explantation | Indication for explantation | Presentation | Technique | |
|---|---|---|---|---|---|
| I | AAA | 72 | AEF | Emergent | Aortic ligation |
| II | rAAA | 63 | AEF | Urgent | Aortic ligation |
| III | AAA | 32 | AEF | Emergent | Incomplete explantation + incorporated traditional bypass |
| IV | rAAA | 24 | AEF | Emergent | Aortic ligation |
| V | AAA | 44 | Type Ia EL + aneurysm expansion | Elective | Complete explantation + traditional bypass |
| VI | rAAA | 16 | Rupture | Emergent | Incomplete explantation + incorporated traditional bypass |
| VII | rAAA | 32 | Infection | Urgent | Aortic ligation |
| VIII | AAA | 19 | Type I EL + Rupture | Emergent | Complete explantation + traditional bypass |
| IX | AAA | 41 | Rupture | Emergent | Complete explantation + traditional bypass |
| X | rAAA | 0 | Type Ia EL after rupture | Elective | Complete explantation + traditional bypass |
| XI | AAA | 3 | Infection | Urgent | Aortic ligation |
| XII | rAAA | 17 | AEF | Urgent | Complete explantation + traditional bypass |
| XIII | rAAA | 12 | Type II EL + aneurysm expansion | Elective | Complete explantation + traditional bypass |
AAA: Abdominal Aortic Aneurysm (elective); rAAA: Ruptured AAA; AEF: Aortoenteric Fistula; EL: Endoleak.
Out of the 11 patients operated primarily at our institution, 3 endoleaks were present at the time of primary intervention, with two being type 2 endoleaks, and one being a type 1 endoleak. One of the type 2 endoleaks and the type 1 endoleak ultimately resulted in explantation. None of these patients was submitted to a previous attempt at endovascular salvage.
Both patients referenced from other hospitals presented with ruptured aneurysms, and we could not ascertain if any endoleak had been present at the time of primary surgery or during follow-up. One of the patients was treated with re-EVAR with an aorto-uni-iliac endograft and the other with explantation. The re-EVAR was ultimately submitted to explantation for aorto-enteric fistula 18 months later.
All but one patient who presented with EVAR rupture or endoleak with sac expansion were in compliance with the respective IFUs (Table 2).
Table 2 Presentation with endoleak (EL) or rupture.
| Indication for explantation | Endoleak in first procedure | Months to explantation | Compliance with IFUs | Pre-operatoy neck diameter (mm) | Neck meter at explantation (mm) | Pre-operatory aneurym diameter (mm) | Aneurysm diameter at explantation (mm) | |
|---|---|---|---|---|---|---|---|---|
| V | Type Ia EL + aneurysm expansion | No | 44 | Yes | 20 | 25 | 59 | 69 |
| VI | Rupture | No | 16 | Yes | 27 | 32 | 57 | 60 |
| VIII | Type Ia EL + Rupture | No | 19 | Yes | 27 | 44 | 98 | 140 |
| IX | Rupture | - | 41 | - | - | - | - | - |
| X | Type Ia EL after rupture | Type I | 0 | No (neck length) | 24 | 52 | 24 | 52 |
| XIII | Type II EL + aneurysm expansion | Type II | 12 | Yes | 26 | 28 | 71 | 180 |
Patient IX was transferred from another hospital and as such we had no information on previous interventions or follow-up. Patient X was submitted to emergent EVAR for rAA and after stabilization, to EVAR explantation. IFU: Instructions for use.
Among the patients presenting with secondary aorto-enteric fistula, the primary intervention had been for rAAA in 60% of patients. Three patients presented with acute blood loss and hemodynamic instability and the remaining two presented with recurrent lower gastrointestinal blood loss.
Two patients presented with graft infection, one with positive haemocultures for Salmonella, and a history of recurrent diarrhoea, and the other presented with severe weight loss and positive haemocultures for Streptococcus anginosus.
None of the patients had lost follow-up (excluding the two patients that were transferred from other hospitals) although the exact timing of follow-up exams and type of exam requested varied widely among physicians.
All patients were submitted to transperitoneal approaches, and all needed initial supracoeliac (five patients) or suprarenal (eight patients) aortic clamping. The exact duration of clamping could not be obtained from patient charts.
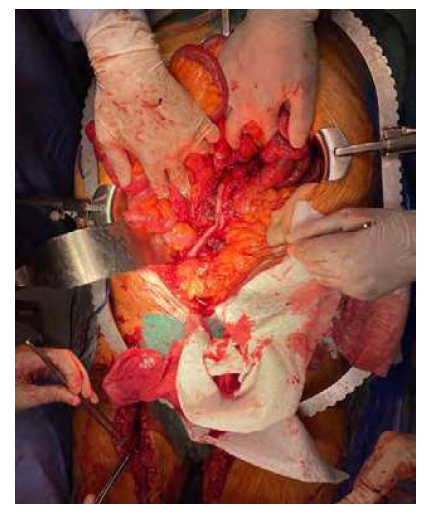
After explantation, in situ reconstruction was performed in eight patients, six of which with complete EVAR explantation and two with partial EVAR explantation. Two in situ reconstructions were made using superficial femoral veins (figure 2), and the remaining used prosthetic grafts. Aortic ligation with cerclage of the infrarenal aorta and reinforcement of the aortic stump with bovine pericardium patch and extra-anatomic bypass were performed in five cases (both infection cases and three of the AEF cases).
Post-operative complications could not be predicted from presentation or type of surgery, and consisted of prolonged ventilation in two cases, renal insufficiency with permanent need for dialysis in two cases, visceral ischemia with need to return to the operating room in two cases, and septic shock in one case.
There was one intra-operative death, for an aorto-enteric fistula, from haemorrhage from the aortic stump. The 30-day mortality was 54% (seven patients) with 33% of mortality for elective repair, 50% mortality for urgent repair, and 67% mortality for emergent repair.
Considering presentation, AEF and rupture had the highest mortality with 60% mortality in each group, graft infection had 50% mortality and endoleak without rupture had a 33% mortality rate.
Considering type of surgery, complete EVAR explantation and in situ reconstruction had 33% mortality, partial EVAR explantation had 50% mortality, and aortic ligation had 83% mortality. Both patients submitted in situ reconstruction with superficial femoral veins survived and were ultimately discharged home.
Mean hospital stay after surgery was 48 days for survivors. Mean survival after discharge was 10 months. The younger patient (47 years old) has the longest follow-up after discharge so far (27 months), with three patients still alive. Age was not a significant factor for survival, as were not pre-existing comorbidities.
Discussion
EVAR explantations are increasing, with reports of 1.9% explantations per year in 2010 to 5.4% in 2018.3 This is probably related to more EVAR procedures being performed, more complex anatomies being considered for endovascular treatment, and greater follow-up time on previous interventions.
The low rate of explantations in our centre (<2%) is in line with what is described in literature for high volume centers.1Notably 54% of the cases of explantation occurred in patients who had been submitted to emergent EVAR for ruptured infrarenal aortic aneurysms, which might mean that either the underlying disease or the nature of emergent repair (when durability of repair is often a secondary consideration) play a role in later complications, despite survival in the acute setting. The mean time for explantation (29 months) was shorter than generally described in literature (approximately 40 months for graft failure or disease progression).
Age, sex and comorbidities are in line with the general vascular surgery population, and with previous publications on this subject, and no specific predisposing factor can be identified from such a small sample of patients. There is however a high proportion of patients with hypertension, as also described in other series.3
The predominance of Medtronic® endografts explanted does not seem to us to be device-related and is possibly explained by availability on an of the shelf basis for the emergency setting in our centre, and as such having a larger volume of patients being treated with these devices. The high proportion of patients submitted to suprarenal and supraceliac clamping can also be supported by the fact that most grafts used have suprarenal fixation.
The small proportion of patients in this series submitted to explantation for endoleak without rupture (two patients, one endoleak type I and one endoleak type II) probably accounts for a significant difference in mortality results when comparing to similar studies for EVAR explantation. Most of such studies exclude aorto-enteric fistulas and infection, because of the underlying multisystemic implications, that can have conflicting results when trying to evaluate different surgical techniques. In our centre the primary treatment modality for type I endoleaks remains endovascular management whenever possible. Patient V (type Ia EL with aneurysm expansion) and patient XIII (type II EL and aneurysm expansion) were however proposed for open surgery as the first modality of treatment. In the case of patient V the previous endograft was a Nellix®, and no endovascular solution was deemed suitable. For patient XIII the initial surgical approach was to ligate the lumbar arteries responsible for the high flow endoleak observed, but ultimately lead to explantation because of the large size of the aortic sac (growth from 71 to 180 mm), that caused the components of the endograft to be displaced with manipulation.
The highest mortality for AEF and rupture does not present as a surprise in this setting. According to literature for endograft infection early mortality rates are 11% to 39% in the best circumstances.
The operative approach was determined mainly by surgeon preference and reason for failure. For infected grafts complete EVAR explantation is recommended and if an anatomic reconstruction is preferred, especially in the elective setting, and for reasonably fit patients, in situ aortic reconstruction with superficial femoral veins might be appropriate.
Complete EVAR explantation and in situ reconstruction seem to carry a survival advantage although the small series and heterogeneity of presentations does not allow for a proper evaluation of the preferable technique to be used and respective results.
The limitations of this study are mainly the small number of patients included and the significant heterogeneity of the sample, that do not allow for a proper evaluation of the variables.
Conclusion
Although EVAR explantation is still a relatively rare event, it is one that will inevitably increase in the near future as the number of EVAR procedures also rise. The heterogenicity of causes that can lead to such intervention, and the lack of inclusion of such causes in many center-based studies, makes for the lack of management guidelines and urgent need for multicentered studies.