Introduction
Atherosclerosis is known to be a systemic and progressive disease affecting multiple territories. Co-existence of atherosclerotic disease has been well described regarding coronary, carotid, and lower limb arterial disease.1 Patients with chronic lower limb ischemia are especially prone to develop concomitant disease in other regions.2),(3 However, studies have focused on co-prevalence of coronary and carotid atherosclerotic disease,1 and little is known regarding the co-existence of occlusive visceral vessel disease.
Patients with coronary disease have been demonstrated to have a high prevalence of synchronous mesenteric occlusive disease. Krishnamurthy et al,4 have shown that in patients undergoing coronary angiography, co-prevalence of mesenteric occlusive disease occurs in 42.7%, with 20.4% presenting with symptoms, or severe stenosis (>70% in more than two vessels). This, however, is not well known when analyzing patients with chronic lower limb ischemia.
Asymptomatic visceral artery disease has been demonstrated to be significantly increased with age, with a described prevalence of significant splanchnic vessel stenosis of 18% in individuals older than 65 years.5 Moreover, these patients may progress to manifest symptoms of chronic mesenteric ischemia or may present as an acute event with a high rate of morbidity and mortality. Studies on the natural history of these asymptomatic patients have shown that 86% of patients with significant but asymptomatic stenosis of all three visceral vessels [Celiac trunk (CT), superior mesenteric artery (SMA) and inferior mesenteric artery (IMA)] will develop mesenteric ischemia or die.6
Since current data regarding the co-prevalence of atherosclerotic disease of the lower limbs and visceral vessels is scarce and visceral occlusive disease, when complicated, may lead to a high mortality rate, we aimed at performing a cross-sectional study to analyze the prevalence of splanchnic and renal visceral occlusive disease in patients admitted for chronic lower limb ischemia (CLLI).
METHODS
This study followed the reporting guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement for Cross-sectional studies.7
Study design, Setting and Participants:
A single-center cross-sectional study based on a retrospective analysis of morphologic imaging using computer tomography angiography (CTA) was performed. Study patients were included from a cohort of chronic limb ischemia patients treated at a Vascular Surgery Department of a central tertiary university Hospital. One-hundred aleatory patients were chosen from the departments database and included in the study. Aleatorization was performed using the Excel “random” function.
In our center, almost every patient that is admitted for treatment due to CLLI is submitted, prior to surgery, to an abdominal, pelvic and lower limb CTA.
Eligibility criteria included all patients who presented with CLLI without previous or current history of mesenteric ischemia, and who were submitted to an abdominal computerized tomography angiography (CTA) as part of the pre-operative work-up.
Exclusion criteria included patients with known history of mesenteric ischemia (either acute or chronic) or with a known previous diagnosis of mesenteric and/or renal artery stenosis. In addition, patients who were not submitted to an abdominal CTA, or in which, one was not available in the hospital records were also excluded from the study.
Only stenosis due atherosclerotic disease was considered. Patients with mesenteric artery dissection or stenosis due to extrinsic compressions such as median arcuate ligament or abdominal masses were excluded.
Variables, Data Sources/Measurement, Bias and Study Size:
All included patients had a triphasic abdominal, pelvic and lower limb CT-angiogram with 1mm slices. All CTAs were analysed with the HOROS® RC5 (version 4.0.0) imaging processing software using multiplanar reconstructions (Figure 1).
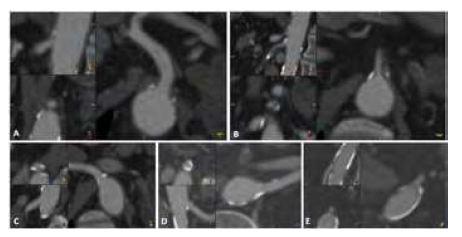
Figure 1: Example of CTA analysis using multiplanar reconstructions for each visceral vessel. A: Celiac trunk; B: Superior Mesenteric Artery; C: Right renal artery; D: Left renal artery; E: Inferior mesenteric artery.
Measurements were performed by an experienced vascular surgeon with training in the processing software. Stenosis was measured as the ratio between the patent inner-to-inner diameter and the outer-to-outer vessel wall diameter. These were assessed in axial, coronal and sagittal view in order to correct the measurements.
The presence of splanchnic (celiac artery, superior and inferior mesenteric arteries) and renal atheromatous disease was defined as mild if the stenosis was between 30 and 50%, moderate if between 50 and 70% and severe if >70% or occlusion was observed. All measured was performed solely by CTA analysis. No additional duplex flow velocities were obtained due to the retrospective nature of the study.
Additional demographic and risk factor variables were collected including age, sex, primary diagnosis, pattern of lower limb atherosclerotic disease, smoking, diabetes, hypertension, coronary artery disease (defined as primary MI, history of angina or myocardial scintigraphy or coronary angiography demonstrating coronary artery disease), COPD, chronic kidney disease and carotid stenosis (defined as >50%).
Study outcomes:
The main outcome was defined and the prevalence of splanchnic and renal visceral occlusive disease. Which was analysed as the prevalence of having any splanchnic artery stenosis; any severe splanchnic artery; 2 or more severe splanchnic artery stenosis; any severe renal disease and bilateral severe renal artery disease. Other outcomes were the evaluation of predictive factors for visceral (splanchnic and renal) occlusive disease and its relationship with the pattern of lower limb atherosclerotic disease.
Statistical Analysis:
Statistical analysis was carried out using STATA version 16.1 (Statistics/Data analysis, StataCorp LLC, Texas, USA). Continuous variables are presented as mean ± standard deviation. Categorical variables are expressed in numbers (percentage). Students t-test was used when comparing continuous variables and the х2 test to compare categorical variables. A logistic regression was performed to analyze possible predictive factors associated with the prevalence of visceral occlusive disease. The following risk factors and possible confounders were included in the model: age, sex, pattern of lower limb disease, smoking, diabetes, hypertension, coronary disease, chronic kidney disease and carotid artery disease. All analyses were considered statistically significant if a two-tailed p-value < 0.05 was observed.
Results
Participants and Descriptive Data:
Overall, 100 patients with CLLI were included in the study. Demographics are detailed in table 1. Mean age was 68.5 years old (SD: 9.7) and 77% of the patients were male. Admission diagnosis was incapacitating claudication (Rutherford stage 3) in 19%, and chronic lower limb threating ischemia (CLTI) in 81%, 21% presented with Rutherford stage 4 (rest pain) and 60% with Rutherford stage 5/6 (ischemic trophic lesions). Seventy-five percent presented aorto-iliac disease (AOID) and 97% presented infra-inguinal disease.
Table 1: Demographics, lower limb atherosclerotic occlusive disease stage and anatomic pattern, and risk factors of the 100 patients included in the study.
| DEMOGRAPHICS | |
|---|---|
| Age [mean (SD)] | 68.5 (9.7) |
| Sex (male) | 77% |
| LOWER LIMB ARTERIAL DISEASE | |
| Infra inguinal | 97% |
| Aorto-iliac | 75% |
| Rutherford 3 | 19% |
| Rutherford 4 | 21% |
| Rutherford 5 or 6 | 60% |
| RISK FACTORS | |
| Hypertension | 82% |
| Type 2 Diabetes Mellitus | 55% |
| Smoking | 75% |
| Coronary artery disease | 82% |
| Dislipidemia | 74% |
| Chronic kidney disease | 25% |
| COPD | 47% |
| Carotid Stenosis >50% | 35% |
SD: Standard Deviation; COPD: Chronic Pulmonary Obstructive Disease.
Prevalence of splanchnic visceral occlusive disease:
Overall prevalence of any visceral disease (mild, moderate or severe) in at least one splanchnic artery was 65%. Severe disease was seen in at least one splanchnic vessel in 60%. Thirty-four percent of patients presented with severe disease in only one visceral artery and 26% presented with severe disease in ≥2 visceral vessels. Furthermore, 22% presented severe disease in all three splanchnic arteries.
Prevalence of renal visceral occlusive disease:
Regarding renal disease, 33% presented severe disease in at least one renal artery. Additionally, 20% presented with bilateral disease.
Association of different stages of chronic lower limb ischemia:
A significant association was found between more advanced stages of CLLI, comparing CLTI with claudicant patients. When analysing any visceral occlusive disease (mild, moderate, or severe) in any splanchnic vessel, the prevalence was higher for CLTI patients (71.6% versus 36.8%), p=0.004. Analysing only severe splanchnic vessel stenosis, CLTI was also associated with higher prevalence (66.7% versus 31.6%), p=0.005. Surprisingly, severe stenosis in ≥2 splanchnic vessels was not found in patients with claudication (0% versus 32%), p=0.004. Severe stenosis in at least one renal artery was also higher in CLTI patients (38.3% versus 10.5%), p=0.021.
Predictive factors associated with higher prevalence of occlusive visceral atherosclerotic disease:
A logistic regression including age, sex, pattern of lower limb disease, smoking, diabetes, hypertension, coronary disease, chronic kidney disease and carotid artery disease was performed. Predictive factors for the presence of at least one severe splanchnic artery stenosis were the presence of aorto-iliac disease, with an OR of 5.42 (95%CI: 1.54; 19.00, p=0.008); and the presence of coronary artery disease, with an OR of 3.89 (95%CI: 1.10; 13.75, p=0.035) - table 2. Predictive factors for the presence of the presence of ≥2 severe splanchnic artery stenosis was age with an OR of 2.01 (95%CI: 1.04; 3.89, p= 0.039) for every 10-year difference and the presence of aorto-iliac disease, with an OR of 14.61 (95%CI: 1.83; 116.58, p=0.011) - table 3. Regarding the presence of at least one severe stenotic renal artery, associated predictive factors included: age with an OR of 3.90 (95%CI: 1.82; 8.36, p<0.001), for every 10-year difference; aorto-iliac disease with an OR of 25.6 (95%CI: 2.75; 238.4, p=0.004) and carotid artery disease with an OR of 9.24 (95%CI: 1.98; 43.06, p=0.005) - table 4.
Table 2: Logistic Regression for one or more renal artery stenosis.
| Logistic Regression for at least one severe splanchnic artery stenosis | |||
|---|---|---|---|
| Variable | Odds Ratio | 95%CI | P |
| Age (every 10-year increase) | 1.18 | 0.65-2.14 | 0.58 |
| Aorto-iliac disease | 5.42 | 1.54-19.01 | 0.008 |
| Smoking | 0.41 | 0.10-1.78 | 0.24 |
| Type 2 diabetes mellitus | 0.75 | 0.25-2.24 | 0.60 |
| Hypertension | 2.15 | 0.57-8.11 | 0.26 |
| Coronary artery disease | 3.89 | 1.10-13.75 | 0.035 |
| Hyperlipidemia | 0.30 | 0.08-1.08 | 0.06 |
| Chronic Kidney Disease | 0.85 | 0.22-3.24 | 0.81 |
| COPD | 0.52 | 0.16-1.64 | 0.26 |
| Carotid Disease | 3.04 | 0.92-10.05 | 0.07 |
| Male sex | 0.76 | 0.23-2.57 | 0.66 |
COPD: Chronic Pulmonary Obstructive Disease.
Table 3: Logistic Regression for one or more renal artery stenosis.
| Logistic Regression for two or more severe splanchnic artery stenosis | |||
|---|---|---|---|
| Variable | Odds Ratio | 95%CI | P |
| Age (every 10-year increase) | 2.01 | 1.04-3.89 | 0.039 |
| Aorto-iliac disease | 14.61 | 1.83-116.6 | 0.011 |
| Smoking | 0.29 | 0.05-1.65 | 0.16 |
| Type 2 diabetes mellitus | 0.67 | 0.19-2.41 | 0.54 |
| Hypertension | 4.68 | 0.43-50.68 | 0.20 |
| Coronary artery disease | 3.23 | 0.76-13.69 | 0.11 |
| Hyperlipidemia | 0.25 | 0.05-1.19 | 0.08 |
| Chronic Kidney Disease | 1.20 | 0.31-4.61 | 0.79 |
| COPD | 0.88 | 0.21-3.64 | 0.86 |
| Carotid Disease | 3.15 | 0.75-13.16 | 0.12 |
| Male sex | 0.36 | 0.09-1.43 | 0.15 |
COPD: Chronic Pulmonary Obstructive Disease.
Table 4: Logistic Regression for one or more renal artery stenosis
| Logistic Regression for one or more renal artery stenosis | |||
|---|---|---|---|
| Variable | Odds Ratio | 95%CI | P |
| Age (every 10-year increase) | 3.90 | 1.82-8.36 | <0.001 |
| Aorto-iliac disease | 25.62 | 2.75-238.38 | 0.004 |
| Smoking | 0.93 | 0.15-5.85 | 0.94 |
| Type 2 diabetes mellitus | 0.51 | 0.14-1.90 | 0.32 |
| Hypertension | 0.82 | 0.14-4.92 | 0.83 |
| Coronary artery disease | 3.71 | 0.91-15.03 | 0.07 |
| Hyperlipidemia | 0.70 | 0.15-3.26 | 0.64 |
| Chronic Kidney Disease | 2.89 | 0.62-13.40 | 0.17 |
| COPD | 0.37 | 0.09-1.58 | 0.18 |
| Carotid Disease | 9.24 | 1.98-43.06 | 0.005 |
| Male sex | 1.19 | 0.24-5.92 | 0.83 |
COPD: Chronic Pulmonary Obstructive Disease.
Discussion
The main findings of this study were: 1) the overall prevalence of visceral disease (mild, moderate or severe) in patients admitted for CLLI was 65%; 2) Severe disease (>70% stenosis or occlusion) was seen in at least one vessel in 60%; and 3) 34% of patients presented severe disease in only one visceral artery, 26% presented in ≥2 visceral vessels and 22% presented severe disease in all three splanchnic arteries.
Seeing as the natural history of patients with occlusive disease of visceral vessels may be dauting, especially in patients with three vessel disease, with complication rate as high as 86% during follow-up6, the high prevalence of severe disease brings some concern and merits future studies to analyze the follow-up outcomes of these patients. Since this was a cross-sectional study, no longitudinal data was collected regarding re-admissions, interventions, or deaths due to mesenteric ischemia.
Furthermore, we also found severe renal artery occlusive disease (stenosis >70% or occlusion) to be also present in 33% of patients, and 20% with bilateral disease. In our cohort, we did not find an association between hypertension and renal artery stenosis. However, this may be due to the high overall prevalence of hypertension in the entire cohort of patients, in fact, in our study, HTA was a risk factor in 82% of patients.
We also found predictors in patients with CLLI, which might indicate higher odds of having synchronous splanchnic and renal occlusive disease.
Patients with CLTI were found to have a have a higher prevalence of mesenteric and renal artery occlusive disease. This is not surprising as we understand that patients with CLTI present with more advance stages of atherosclerosis and usually have more pronounced risk factors.3
We also found age, aorto-iliac disease, and co-prevalence of coronary artery disease to be associated with higher odds of having severe splanchnic artery disease. This worsens the overall prognosis of these patients, seeing as they are the ones with higher risk of developing mesenteric ischemia, but are also the most fragile and less prone to survive and event. In addition, we also found age and carotid artery stenosis to be associated with a higher prevalence of renal artery disease.
This apparent link between carotid and renal artery disease and coronary and splenic disease in patients with CLLI might be conditional of certain patterns of atherosclerosis which we are still unaware. Possibly, a tropism for certain regions in different types of patients, with different manifestations of atherosclerosis may occur. Future studies would be needed to further investigate this hypothesis.
This study has some limitations. The retrospective nature of the study enhances the risk of recall and reporting bias. Although patients were chosen randomly from a cohort of CLLI patients, the dependence on having an adequate CT, absence of extra-vascular stenosis or dissections might increase the risk for selection bias.
In addition, measurements were performed using CTA which may overestimate calcified lesions, and no data were collected regarding duplex artery velocities and wave patterns to assess flow. Also, measurements were performed by one investigator which may increase the risk of systematic error.
Future studies are needed to assess the natural history of these patients and what is the impact of these findings. Since we did not analyze the impact of these visceral occlusive diseases it is difficult to propose any kind of screening without performing further studies. Overdiagnosis may also carry some extra burden for patients including more exposure to radiation, unnecessary and possibly overtreatment and anxiety with their findings. Studies analyzing screening for asymptomatic atherothrombotic disease in patients with severe coronary disease have not proven to be beneficial.8 However, in the setting of chronic lower limb ischemia, this is yet to be analyzed. Furthermore, stratification of risk factor for synchronous occlusive disease, including patients with aorto-iliac disease, coronary artery disease, old age and carotid artery disease, may also be evaluated in order to adjust screening guidelines.
A key message is that co-prevalence of visceral occlusive disease in patients with CLLI is far more common that expected, and thus, its occurrence and diagnosis should not be forgotten and underestimated.
Conclusion
Our study showed a high prevalence of multi-visceral and renal occlusive disease in patients admitted for chronic lower limb ischemia. We found an association between coronary and carotid disease with splanchnic and renal disease, respectively. Age was also associated with more severe stages of visceral and renal artery disease. More studies are needed to analyze the clinical impact of our findings regarding planning and follow-up for these patients.















