Introduction
Endovascular aneurysm repair became the preferred modality for the treatment of ruptured abdominal aortic aneurysms (rAAA).1 Patients with rAAA usually present with significant retroperitoneal hematoma, a space-occupying lesion, which may increase intra-abdominal pressure. Additionally, in the perioperative period, these patients have large fluid requirements from ongoing bleeding, coagulopathy, and third-space requirements that lead to bowel edema and ascites, further increasing intra-abdominal pressure.2,3
Sustained increased intra-abdominal pressure (IAP) leads to abdominal compartment syndrome (ACS) which is defined as intra-abdominal pressure (IAP) greater than 20mm Hg with or without an abdominal perfusion pressure (APP = mean arterial pressure (MAP) - IAP) less than 60 mm Hg that is associated with new organ dysfunction/failure.4 In patients undergoing, r-EVAR, many factors are known to increase the risk for ACS development: aortic occlusion balloon, severe coagulopathy, massive transfusion requirements, pre-operative loss of consciousness, low pre-operative blood pressure and emergent conversion of bifurcated grafts to AUI devices.5,6
Increased intra-abdominal pressure has deleterious effects on multiple organ systems. As intra-abdominal pressure rises, the inferior vena cava is compressed, resulting in a decrease in venous return that in turn leads to reduction in ventricular end-diastolic volume, stroke volume, and cardiac output and elevation of systemic vascular resistance. Elevated intra-abdominal pressure also compresses the kidneys, and this along with the adverse effects on cardiac function causes a reduction in renal flow and a decrease in urine output. Simultaneously, increased intra-abdominal pressure compresses the diaphragm, raising intrathoracic pressure with an increase in airway pressures, pulmonary artery pressure and central venous pressure and a decrease in pulmonary compliance. If ACS is left untreated, multiorgan failure develops.1
ACS is described to occur after EVAR or OR for rAAA. Incidence of ACS after -EVAR is widely described, with incidence rates ranging from 6.6% to 40% and has been associated to a significantly greater risk of mortality.6,7 Treatment is directed at relieving the intra-abdominal pressure, often requiring a decompressive laparotomy. Failure to relieve the pressure is almost uniformly fatal; surgical decompression demonstrates significant improvement in mortality.1
The aim of this study is to overview the incidence and mortality rates due to ACS after r-EVAR along with impact of surgical decompression.
Methods
A literature search was performed to identify studies investigating the incidence and impact on mortality of ACS after EVAR for ruptured abdominal aortic aneurysms. The MEDLINE databases were searched between January 2002 and March 2020.
Studies were included if they met the following criteria: data on human implantations, in English language and include prevalence of ACS patients after ruptured EVAR. Case series with less than 25 patients were not considered. After relevant titles were identified, all the abstracts were read, and eligible studies were retrieved.
Results
Epidemiology:
A significant heterogeneity is observed throughout the literature regarding ACS incidence. Among larger studies (n>100) ACS incidence ranged between 6.9 to 20%.6,8-11 Ersyd et al, in a study including patients from the Swedvasc registry (2008-2013) reported a 6.9% of ACS syndrome after r-EVAR, similarly to those after open repair (OR) - 6.8%. Later the same authors have extended the period of inclusion to 2008-2015, and scrutinized the case records beyond registry data, reporting a ACS rate of 7.5%.8 In another report, including 1241 patients, Adkar et al. identified 1241 patients submitted to r-EVAR from NSQIP (an independently audited and validated clinical database from USA), of which 91 (7%) patients also underwent concomitant laparotomy due to ACS.9 In another publication, among 136 patients submitted to r-EVAR 17 patients had ACS (13%). In the latter, the authors reveal a significantly greater proportion of patients developing ACS if peri-operarive hemodynamic unstable.10 According to Mayer et al., 20 out of 102 patients undergoing r-EVAR (20%) required laparotomy for abdominal compartment syndrome either during intervention (n=14) or after in the ICU (n=6).11 Several other smaller studies have reported ACS incidence after r-EVAR ranging from 3 to 24%.5,12-19
Mortality:
The development of ACS after open or endovascular treatment for rAAAs carries significantly additional mortality risk.6
Yet, heterogeneity is also observed, with reported peri-operative mortality ranging from 30 to 83%. According to Adkar et al, the need for decompressive laparotomy, as indicative of patients with ACS syndrome, carried a significantly greater risk of in-hospital mortality (odds ratio [OR], 5.91; 95% confidence interval [CI], 3.62-9.62;P<.001).9 In accordance, another study showed that patients with ACS had significantly greater in-hospital mortality when compared to patients witouht ACS (10 of 17 [59%] vs 22 of 119 [18%];P<.01).10
Mayer et al. reported a 30-day mortality of 30% (6 of 20) for patients with ACS, when compared to 8% (7 of 82) for patients without ACS.11 According to Rubenstein et al, 5 out of the 6 (83%) patients with post r-EVAR ACS died peri-operatively, while mortality in patients without ACS was 17% (4/23).15 In line, Gidlund et al., in a prospective multicentric study including 29 patients undergoing r-EVAR reported 33% mortality rate (1/3) in ACS patients compared with 10% (3/29) in non-ACS patients.14
Treatment:
As ACS is frequently linked to a fatal outcome, several strategies must be carried out in order to minimize ACS occurrence but also to timely recognize this disorder and take prompt action. Then, in patients undergoing r-EVAR close monitoring of IAP is paramount to permit early diagnosis and rapid abdominal depressurization IAP.20 When IAH/ACS is suspected, at first, non-surgical management should be attempted to reduce IAP. If conservative measures prove unsuccessful and a full blown ACS has developed, surgical decompression is indicated, usually through midline laparotomy.20 Less invasive approaches, such as translumbar extraperitoneal decompression, have been reported, but the safety of these procedures has not been shown.21,22
Medical treatment options for reducing IAP include reducing intraluminal volume/abdominal content through nasogastric and/or rectal tubes or percutaneous catheter drainage (paracentesis), improving abdominal wall compliance through analgesia and/or sedation, neuromuscular blockers and changing body position when indicated, correct positive fluid balance avoiding over resuscitation and crystalloid, institution of diuretics (furosemide) and renal replacement therapy if indicated.23
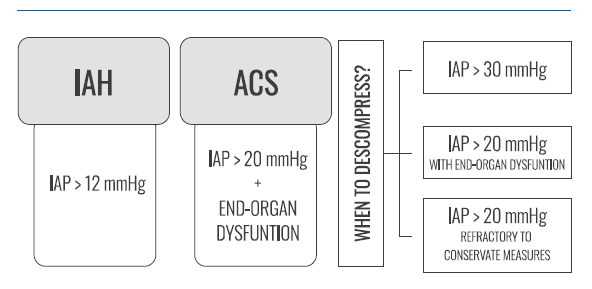
Most patients developing ACS after r-EVAR require expedite surgical decompression in order to improve survival rates. Despite lack of data regarding the impact of decompressive laparotomy on survival, in a study including 102 r-EVAR patients decompressive laparotomy was performed when intravesical pressure >20 mmHg or when abdominal perfusion pressure was <50 to 60 mmHg and concomitant organ deterioration. All the 20 patients diagnosed with ACS were submitted to DL. Among these, 14 patients performed DL intra-operatively, while the remaining latter in the ICU. The authors reported an in-hospital mortality rate of 30% (6/20).11 According to Mehta et al., six out of the 30 patients undergoing EVAR developed ACS and required DL. Among those six patients undergoing DL, four patients died (67%) of multiorgan failure, while the two survivors had considerable post-operative morbidity, but survived over 13 months of follow-up.5 Gidlund et al, also had close post-operative monitoring after r-EVAR with IAP monitoring very 4h during the first 48 post-operative hours. Early conservative treatments (pain relief, diuretics, colloids and neuromuscular blockade) were given to patients with IAP > 12 mmHg while decompression laparotomy was performed when ACS developed (IAP >20 mmHg and new organ dysfunction). Among the 29 monitored patients, six had an IAP >20 mmHg (21%) of whom three were successfully treated conservatively and three (3/29 - 10%) developed ACS. Of those with ACS, one patient was old and dependent and no laparotomy was offered, and one patient died. The remaining two were offered a DL and survived.14 Indications for surgical decompression are summarized in Figure 1.
Discussion
EVAR of rAAA has gained popularity due to decreased morbidity and mortality. However, there is an increased recognition of complications, such as ACS.24 ACS is a major cause of death after endovascular for RAAA reaching up to 83%.11,15 Therefore, early detection and expedite treatment of ACS are, therefore, considered lifesaving and mandatory.25,26) Patients undergoing r-EVAR are inherently spared a laparotomy incision. Aggressive fluid resuscitation is often required, which make this subset of patients vulnerable to ACS due to splanchnic oedema and space occupying hematoma.9 When IAP>20 mmHg there is a decrease in the arterial flow and venous drainage in all the abdominal organs. The first organ affected is usually the kidney.8) Consequently, renal dysfunction is the most common form of presentation. Additionally, ACS results in ischemia-hypoxia at the abdominal level, produces dramatic changes in cellular membranes, favoring interstitial oedema and consequent bacterial translocation which ultimately leads to multi-organ failure (MOF) in ACS.27
Reported incidence of ACS is significantly heterogeneous probably attributable to different post-operative surveillance protocols. Among the included studies, incidence of ACS varied between 3 to 24%, with greater variations among smaller studies, as expected.5,12,19 Among publications with at least 100 patients undergoing r-EVAR, a smaller heterogeneity was observed - from 6.9 to 20%.6,8,11) The greatest incidence was reported by Horer et al. - 40%.7 In the latter report, 16 r-EVAR patients were post-operatively monitored hourly for IAP, urine production and, through micro-dialysis, intra-peritoneal lactate, pyruvate, glycerol and glucose, potential early markers of IAH. Consequently, as a more detailed surveillance protocol was adopted, significantly higher rates of ACS were described in this study. The latter finding also reinforces that true ACS incidence is, most likely, underestimated. Besides, different incidence of reported ACS incidence may also be dependent on the patients’ state at the time of repair and repair method.
Most frequently, ACS syndrome develops shortly after procedure - greater incidence among 8h hours after EVAR. Intra-operative unsolved endoleaks may drain the wall rupture and further increase IAP.27 Consequently, in the rupture setting a more aggressive approach with type 2 endoleaks may be reasonable in order to avoid ACS - with embolization or surgical ligation of lumbar arteries and inferior mesenteric arteries.
The impact of ACS on early mortality after r-EVAR is firmly described with mortality rates reaching up to 83%.15 In a large study, Adkar et al, identified in the NSQI database that 91 out of 1241 (7,3%) of patients undergoing r-EVAR in some institutions of the US from 2005 to 2013 required additional laparotomy as a proxy of ACS. In this study, 30-day mortality was 60% in the ACS group and 21% in the non-ACS group (P<.001). Yet, patients who received a concurrent laparotomy more frequently required preoperative ventilation or preoperative transfusion of blood products. Besides, hemodynamic status at the time of repair was also different with increased prevalence of shock (4% vs 2%) and systemic inflammatory response syndrome (35% vs 23%) in the group with concurrent laparotomy. However, after correcting for potential confounders, the need for laparotomy increased by six-fold the risk of 30-day mortality.9 Also Mehta et al., in a study including 136 r-EVAR patients showed a greater risk of in-hospital mortality when compared to patients without ACS (10 of 17 [59%] vs 22 of 119 [18%]; P<.01).
ACS related to rAAA is observed both for EVAR and OR.26,28 Yet, Rubbenstein et al. reports higher mortality rates among ACS patients undergoing EVAR compared to those with ACS after OR.15 In patients treated for rAAA with an open operation, intra-abdominal hypertension appears to be the result of fluid resuscitation: shock state contribute to third-space fluid requirements, often resulting in the need for massive fluid administration. Shock and hypothermia can result in coagulopathy, increasing the need for blood and blood products. Patients who developed ACS after EVAR received significantly more blood, blood products, colloid, and crystalloid intraoperatively compared with either those patients who had EVAR without the development of ACS or patients who developed ACS after open repair. This implies that there was significantly more blood loss occurring during the operation in the EVAR/ACS patients and is consistent with the higher estimated blood loss in this subset of patients.15Permissive hypotension is proposed as a potential method for avoid ACS. Typically referred to as the maintenance of systolic pressure <70 mm Hg through delayed or minimized fluid resuscitation, it may reduce abdominal bleeding along with intestinal oedema. Studies to date have investigated the efficacy of permissive hypotension in the setting of hemorrhagic shock.29-31 Although animal studies have demonstrated a benefit of permissive hypotension in models of ruptured aneurysms,32,33 clinical studies assessing the efficacy of permissive hypotension in the context of REVAR have been scarce.
Given the greater mortality linked to ACS development, thigh monitoring and decompression are paramount for patients’ survival. Despite there are non-invasive approaches for diminishing IAP, when ACS is established, surgical decompression is needed to avoid organ ischemia and consequent failure. Despite lack of studies reporting the magnitude of importance of DL on ACS-related survival, one can notice that mortality without decompression rounds 100%, which is presumptive of a demand for a rapid surgical action.
The present review has several limitations. First, it is not a systematic review and consequently there is risk that not all data have been captured. Second, most data comes from single centre studies increasing risk of selection and publication bias. Also, it was difficult to quantify the impact of decompressive laparotomy, due to unacceptable risk of not carrying out such procedure. Finally, due to different post-operative surveillance strategies, one could find significant heterogeneity in ACS rates, highlighting for presumptive underdiagnose of this condition.
Conclusion
ACS syndrome represents a common and highly fatal event after EVAR for ruptured abdominal aortic aneurysm. Prompt recognition and surgical decompression are lifesaving. Therefore, close intra-abdominal monitoring shortly after EVAR and expedite decompressive measures are recommended to improve survival.