Introduction
Endovascular aneurysm repair (EVAR) has become a current standard of care for abdominal aortic aneurysms (AAA). Continuous development in endovascular treatment of aortic aneurysms, together with growing experience and encouraging results, have opened the way for a new generation of stent-grafts.1 However, the long-term success is limited by complications, most importantly endoleaks, that are associated with high reintervention rates. In case of a type Ia endoleak, which represents failure of achieving an adequate proximal seal, secondary intervention is strongly advocated to prevent secondary aneurysm rupture. Different treatment options are suggested such as endoanchors, parallel grafts like chimneys, fenestrated cuffs, embolization, or open conversion.2,3 A type Ia endoleak detected in the operative room can be promptly treated with adjunctive procedures.4 However, a delayed onset of this type of endoleak can reflect progression of the aneurysmatic disease. Dilatation of the proximal neck can occur, with expansion of the aneurysm, eventually resulting in a juxtarenal or pararenal AAA. Endovascular treatment of type Ia endoleaks secondary to aortic neck dilatation can raise many technical challenges related to the previous implanted stent graft.5 Catheterization difficulties are expected due to passage through the limbs or struts of the previous stent graft, in particular in the case of a previous graft with supra-renal fixation stent whose struts can challenge renal catheterization.12
On another hand inadequate working distance related with a short length from renal arteries to previous implanted EVAR bifurcation can increase the complexity of redo endovascular cases and may require creativity and flexibility in the design and deployment of these new devices.13
We describe a case where a physician-modified Zenith® fenestrated stent graft and two parallel aortic covered stents were used in reparing a delayed type Ia endoleak.
Case report
An 84-year-old man with a past medical history of smoking, atrial fibrillation, acute ischemic stroke, hypertension and dyslipidemia initially underwent an EVAR for a 55mm infrarenal AAA (with a straight neck of 22mm of diameter, without thrombus or calcification and a good landing zone) with a TREO Bolton® endograft.
The patient did not attend the next appointment after hospital discharge, and he was lost for follow-up. Three years after the initial EVAR, a CTA scan was performed showing a delayed type Ia endoleak secondary to aortic neck dilatation with significant growth of the aneurysm sac, with a maximum diameter of 9.8 cm (Figures 1 and 2). The patient remained asymptomatic.
An endovascular proximal extension was planned, using a fenestrated cuff (ZFEN platform, Cook Medical, Bloomington, Ind) but the short distance from the top of the fenestrated cuff to the previous EVAR bifurcation did not allow the implantation of a standard Zenith® CE fenestrated stent graft (94cm stent graft length; top of ZFEN fabric to previous EVAR bifurcation 78cm length). To overcome this challenge, an on-table modification of the fenestrated stent graft (with 1 large strut fenestration for the SMA, 2 small fenestrations for the renals) was planned by cutting the distal aortic stent. Also, to overcome the short working distance from the lowest renal artery to the previous EVAR bifurcation, a parallel stent graft configuration using two aortic covered stents sealed in the fenestrated cuff and in each iliac limb of the previous EVAR was proposed.
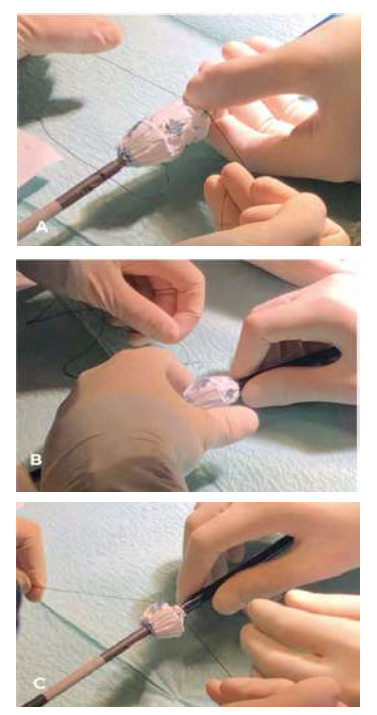
The fenestrated endograft was partially deployed on-table, the distal stent was cut with thermocautery, and the device was re-sheathed. (Figures 3, 4)

Fig 3 A and B: Fenestrated stent graft partially deployed on-table and cutting the distal stent with thermocautery.
Under general anaesthesia, percutaneous bilateral ultrasound guided access of the common femoral arteries was obtained. The fenestrated cuff was implanted in the standard fashion with target vessel catheterization and renal stenting (BeGraft® Bentley InnoMed GmbH 6mm). Two aortic covered stents (Begraft Aortic®stent Bentley InnoMed GmbH 18mmx48mm) were implanted inside each iliac limb of the previous EVAR and sealed proximally in a parallel graft configuration on the fenestrated cuff.
The final completion angiogram demonstrated perfusion of bilateral renal arteries, resolution of the Ia endoleak and without further endoleaks, as well as perfusion of both hypogastric arteries (Figure 5). At two months of follow-up, the patient remains asymptomatic and the angio-CT scan showed resolution of type Ia endoleak (figure 6) and the presence of a late type II endoleak.

Fig 5: Final completion angiogram. Deployed modified Zenith® CE Fenestrated Stent Graft with patent renal and visceral arteries and absence of endoleaks.

Fig 6: Two-month post-operative follow-up CTA. A: Three-dimensional reconstruction of the Fenestrated Stent Graft with patent stents and resolution of type Ia endoleak; B: axial fenestrated stent graft at the level of the renal stents; C: Kissing aortic covered stents in parallel graft configuration)
Discussion
Endovascular aneurysm repair has become the first-line treatment modality for infrarrenal aortic aneurysms.1 However, obtaining successful long-term results usually requires new interventions. While rarely some type I endoleaks may resolve spontaneously during follow-up, usually require prompt reintervention because of the risk of aneurysm expansion and rupture.7 Delayed type Ia endoleaks require challenging interventions, because they are frequently associated with proximal extension of the aneurysmal process to juxtarenal or pararenal aortic segments.6
Many endovascular techniques are available to address the treatment of type Ia endoleaks. The proximal extension with a fenestrated stent graft (FEVAR) is one of the definite solutions for the repair of juxtarenal abdominal aortic aneurysm after prior EVAR failure.10,11 This was the preferred treatment approach chosen for the described case, that considered factors as age, clinical and anatomic characteristics and time required for graft customization. In this case, the proximal neck was a straight neck, without thrombus or calcification with a good landing zone, thus with a theoretically expected lower probability of proximal extension of the aneurysmal aortic degeneration.
FEVAR after EVAR can raise many technical challenges such catheterization difficulties or the presence of an inadequate working distance to previous implanted EVAR bifurcation.10,11 When this working space is not available, the usual alternative is a branched endograft, that brings the disadvantages of a more extensive proximal aortic coverage. To overcome this issue, it is described the successful off the shelf usage of a physician modified ZFEN stent graft whose length was adapted to the patient anatomy by cutting the distal stent on table. In the described case considering ZFEN platform, the short fenestrated cuff available with two proximal internal sealing stents was 94cm length. This was modified to be shorter by cutting the distal stent to adapt to patient’s anatomy measurements (ZFEN top fabric to the previous EVAR length 78cm). We decided to use two balloon-expendable covered stents - implanted inside each iliac limb and sealed proximally in a parallel graft configuration in the fenestrated cuff - instead of self-expendable stents because of a better deployment and alignment with the renal stents of the FEVAR. These two stents were implanted to improve the extension of the landing zone and eventually the durability.
Another frequent technical issue encountered during FEVAR after EVAR failure is the insufficient distal seal in the previous stent graft and the lack of adequate working length required for complete relining with a fenestrated cuff and bifurcated device, especially with previous devices featuring a short body.12 This potential short working length from the lowest renal artery to the previous EVAR bifurcation is usually overcome with a custom made bifurcated stent graft incorporating an inverted limb. To overcome the cost and manufacturing time of this component and as an alternative to achieve the distal seal of the fenestrated stent graft the authors described the use of two parallel covered stents extended to each iliac limb in a kissing like configuration. This can be an alternative to the inverted limb bifurcated stent graft unibody. Another solution for this issue described in the literature is a custom-made 5-branch device (Cook Medical, Bloomington, IN, USA) tapered and featuring an additional posterior branch used for sealing in the contralateral iliac limb.14 However, as already mentioned, branched stent-grafts may require a more extensive aortic coverage, increasing the risk of spinal cord ischemia.14
On-table physician stent-graft modifications have been greatly described in literature, as off-the-shelf ultimate solutions to adjust complex patient anatomies. Most of the reported physician modified stent-grafts describe on-table in situ stent-graft fenestrations for visceral target vessels. In a recent report Paludetto et al.15 described a two case experience, in which the use of physician modified off-the-shelf endografts appears to be a feasible and effective alternative to fenestrated endovascular repair in patients with juxtarenal abdominal aortic aneurysms at high risk for open surgical repair. In 2012, Starnes W. B. conducted a retrospective, nonrandomized, single institution evaluation of the safety and efficacy of physician modification of a currently Food and Drug Administration-approved device to preserve branch vessels when used in the treatment of patients with elective, symptomatic, or ruptured juxtarenal aortic aneurysms.16 In this study, the investigators followed 47 consecutive patients underwent fenestrated endovascular repair using physician modified fenestrated stent grafts over a 3-year period. On follow-up, six patients (13%) had endoleak. There was one type 1 endoleak and five type 2 endoleaks. There were two deaths in the first year, one in the second year, and zero in the most recent year of experience. One patient with endoleak (2%) had aneurysm sac expansion at 1 year requiring secondary intervention. They concluded that physician-modified endovascular grafts are a safe and effective alternative for treating patients with juxtarenal aneurysms who have no other alternatives for repair. Longer-term follow-up is needed to assess the durability of repair and potential for device-related complications It is the author’s that device modification should be performed only at centers completely familiar with all advanced endovascular aortic and visceral artery techniques and a high volume of these fenestrated procedures, as in our hospital centre.16
In conclusion, we report a successful advanced technique using an off-the-shelf physician modified fenestrated stent graft to repair a delayed type Ia endoleak and maintain renal perfusion in a patient with a previous implanted stent graft. Careful evaluation of patient anatomy and previous endografts should be done in planning for these procedures. On-table modification of stent grafts is a valid solution to overcome challenging limitations. Short-term results are promising using this approach, but further long-term follow-up is needed to determine the durability and the potential for device-related complications.