Introduction
Chronic iliocaval venous obstruction (CIVO) can condition debilitating symptoms like venous claudication and ulceration, with a poor response to conservative management.1 Endovascular treatment has been established as the first-line invasive therapy due to its low morbidity and good results.2 Open surgical and hybrid procedures are only used as a last resort due to their higher invasiveness and risks.3,4 However, open venous reconstruction procedures should not be discarded from the vascular surgeon's armamentarium as some patients non-responsive to conservative measures are also not amenable to endovascular treatment.
We present two cases of surgical venous reconstruction and discuss the evidence regarding technical aspects to improve long-term patency. Informed consent was obtained from both patients for the use of clinical data.
Case reports
Case 1:
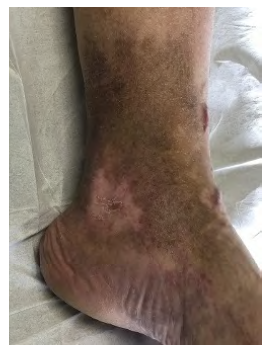
A 69-year-old male was submitted to a surgical correction of a lumbar herniated disc 26 years ago, complicated with a postoperative iliofemoral deep vein thrombosis in the left lower extremity. He was treated with anticoagulation and compression therapy. Post-thrombotic syndrome (PTS) appeared gradually, and after three years, he manifested malleolar ulceration. After failed conservative treatment, the patient underwent balloon venoplasty and stenting of a total occlusion of the left iliac venous axis using two 12mm S.M.A.R.T.® stents (Cordis Corp, Fremont, California, USA). Symptomatic improvement and ulceration healing were observed after treatment. However, stent thrombosis occurred two years later, and the ulceration recurred. Since the time from stent thrombosis was unknown, acute thrombectomy or thrombolysis was not attempted, and the patient was put under conservative treatment. Still, chronic ulceration and venous claudication severely limit the patient's functional status, having a Villalta score of 29. A laborious endovascular recanalization attempt using antegrade femoral and retrograde jugular accesses was unsuccessful (Fig 1).
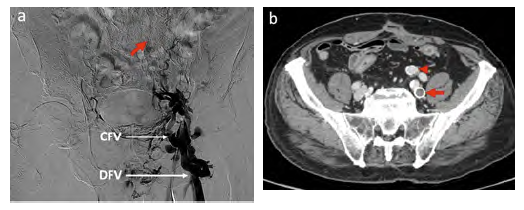
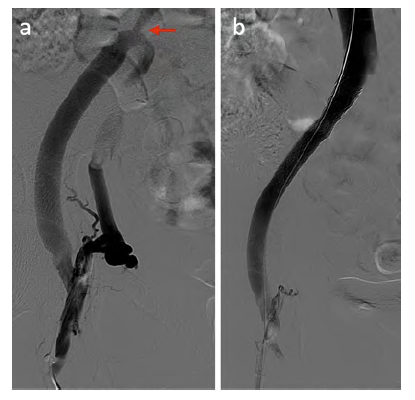
Figure 1 (a) Left lower limb phlebography showing venous stent occlusion (red arrow). (b) Computed tomography venography with patent ringed ePTFE femorocaval bypass (arrowhead) and venous stent occlusion (arrow). CFV - Common Femoral Vein; DFV - Deep Femoral Vein.
So, a femorocaval venous bypass was constructed with a 10mm ringed ePTFE graft (Fig 2). An arteriovenous fistula (AVF) was created with a 5mm expanded polytetrafluoroethylene (ePTFE) graft between the superficial femoral artery (SFA) and the distal segment of the graft. The inferior vena cava (IVC) was isolated through an infraumbilical midline incision. The patient was discharged on postoperative day seven and medicated with lifelong rivaroxaban (20mg daily). After three months, the venous ulcer was completely healed (Fig 3) and a marked improvement in the Villalta score to 14 was observed. A vascular ultrasound showed a patent venous bypass after one year of follow-up and there were no complications during this period.
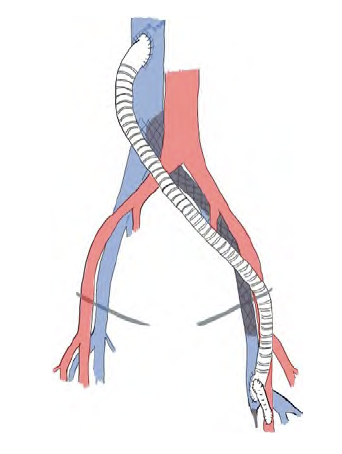
Fig 2 Schematic venous bypass representation from the common femoral vein to the inferior vena cava using a 10mm ringed ePTFE graft.
Case 2:
A 63-year-old female presented with disabling chronic edema of the right lower limb and venous claudication having a previous history of right common femoral vein (CFV) ligation to repair an iatrogenic vascular injury during orthopedic surgery eight years ago. Complete femoral vein thrombosis was confirmed by vascular ultrasound in the postoperative period. Considering the low bleeding risk and outflow obstruction, lifelong anticoagulation was decided.
Venous obstruction symptoms gradually worsened through the following years with significant edema and venous claudication, causing a massive impact on the patient's quality of life (Villalta score of 24). Since an endovascular solution was impossible, the patient underwent a venous bypass between the right deep femoral vein (DFV) and the ipsilateral common iliac vein with a 12mm ringed ePTFE graft. Using the great saphenous vein (GSV), a distal AVF was constructed between the SFA and the distal segment of the ePTFE graft (Fig 4). The patient was discharged 14 days after the procedure and medicated with lifelong rivaroxaban (20mg daily). A sustained improvement in her complaints with a Villalta score reduction to 14 after surgery was observed. Although remaining with sustained symptomatic improvement, a follow-up computed tomography venography (CTV) revealed an ePTFE-iliac vein anastomotic stenosis one year after the procedure. To prolong the bypass patency, she was treated by percutaneous transluminal venoplasty with a 14mm balloon and a Sinus Venous™ stent (OptiMed, Ettlingen, Germany) of the same diameter (Fig 5). At 18 months of follow-up, the graft patency was confirmed by vascular ultrasound. There were no other complications during this period.
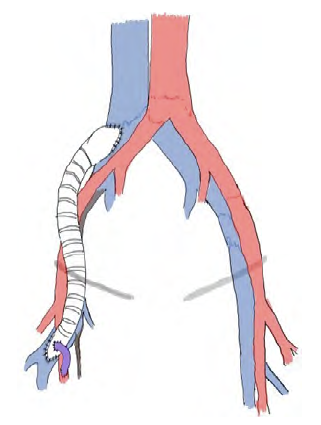
Fig 4 Schematic bypass representation from the deep femoral vein to the common iliac vein using a 12mm ringed ePTFE graft.
Discussion
Iliocaval obstruction associated with trauma, malignancy, or external compression may be better addressed by open surgery.5 However, in most cases of CIVO, surgical reconstructions are reserved for endovascular treatment failure in patients unresponsive to conservative measures but are rarely performed. Both our patients had debilitating symptoms unresponsive to conservative or endovascular treatment, and open surgery was the only alternative.
Different surgical options are available. The femorofemoral crossover bypass (Palma-Dale procedure) is a relatively simple procedure and should be the first option in cases of unilateral iliac disease. Reported primary and secondary patency at five years for vein grafts are 70% and 78%.3,4 Unilateral reconstructions (femoroiliac, femorocaval, or iliocaval bypasses) should be used as an alternative if contralateral iliac disease exists or, in short, segmental vein lesions as reduced-length grafts have better patency.4 In the presented cases, we opted for unilateral reconstructions due to the contralateral disease at the iliocaval junction in the first patient and short segmental occlusion in the second. Reported outcomes are limited to small series. For femoroiliac and iliocaval bypasses, reported five-year patency rates are 63% and 86%, and for femorocaval bypasses, 31% and 57%.3,4
Attention to technical details is important for improved patency of surgical and endovascular venous reconstructions. Healthy inflow and outflow veins are ideal. Infrainguinal venous disease with post-thrombotic occlusion and valvular reflux reduces bypass success.3 In our patients, we wanted to guarantee adequate inflow, which led us to use the DFV for inflow in the second case.
Autologous grafts have the best long-term outcomes and the advantage of reduced risk of infection. Usually, the conduit of choice is the GSV because of its low thrombogenicity and easy access.6,7 It is possible to spiral the graft to fit large-diameter veins. When the GSV is not available, the use of the contralateral femoral vein could be considered but with a higher risk of morbidity. Prosthetic grafts are an alternative when a suitable autologous vein is not available. Due to low flow velocity and luminal pressure, grafts in the venous system are more prone to collapse and thrombose. Ringed ePTFE grafts allow support from external compression and are considered the best prosthetic graft for large vessel replacement.5,8 In both patients, our first option was for a ringed ePTFE graft since compression at the inguinal ligament was could occur..
There is no consensus on using a distal AVF. Still, it seems to improve the patency of prosthetic grafts in the venous system.9 Important aspects to avoid high flow, risking increased venous pressure, arterial or venous dilation, and high-output heart failure (HOHF) should be considered.4 Intraoperative ultrasound can measure AVF flow to avoid more than 300ml/min.8 In our patients, we opted for long-term AVF since no symptoms of HOHF occurred. If necessary, our approach will be the endovascular exclusion of the AVF with a vascular plug.
Optimized management of perioperative anticoagulation, compression pumps, and postoperative surveillance can improve long-term patency.1 In our center, the postoperative protocol includes systemic anticoagulation with UNH combined with intermittent pneumatic compression until ambulation and then lifelong anticoagulation with a direct oral anticoagulant; however, no robust evidence exists.
Graft surveillance with ultrasound is recommended at 3,6 months, and then twice a year.3 Our case demonstrates the importance of detecting graft, vein, or anastomotic stenosis that could be addressed with endovascular intervention to improve long-term outcomes.9
Conclusion
Although it cannot be generalized, our experience supports open venous reconstruction as a viable option for patients with benign ICVO with debilitating symptoms not amenable to endovascular treatment. AVF seems to improve long-term patency and should be used primarily if a long-length graft is needed. Our cases highlight the importance of patient selection, preoperative planning phlebography, the attention to technical details of the procedure and long-term anticoagulation for better outcomes. Adequate follow-up should be maintained to detect and treat possible stenotic lesions.