INTRODUCTION
After basal cell carcinoma (BCC), the second most common malignancy is cutaneous squamous cell carcinoma (CSCC).1,2Its incidence has continued to rise, with estimates that it has increased from 50% to 300% in the last thirty years, and that in the next decade, its incidence is predicted to double.3-5
For Caucasians, the risk of developing a CSCC in a lifetime is 7% to 11%.6 The risk is much higher if we take into account, solid organ transplant recipients (SOTR), who have a 65-250-fold greater risk.7,8In contrast, the incidence of basal cell carcinoma is only 6-10 fold higher in SOTR.9,10
Consequently, instead of the normal BCC/CSCC ratio of 4:1 in the general population, this ratio has been reported as being inverted in SOTR, with CSCC being four times more common than BCC.8
In comparison with renal or liver transplant recipients, heart or lung transplant recipients have been found to have an even higher incidence of CSCC.10 This incidence is probably due to more intensive immunosuppressive regimens required after heart or lung transplants as well as the older age of transplanted patients.9
For localized CSCC, the ten-year-survival after surgery surpasses 90%, but these tumors can be locally invasive or even metastatic, with rates of lymph node metastases of about 4% and mortality rates of almost 2%.11,12In SOTR, not only are CSCC more frequent, but they are also more aggressive, with greater tumor depth, higher rates of recurrence and higher rates of local and distant invasion.13,14
PORTUGUESE REALITY
In the last 10 years, four reviews about cutaneous malignancies in SOTR were published.15-18 Data about these studies is summarized in Table 1. In these publications, the number of transplant recipients ranged from 127-319 patients, with a mean age of 50.7-63.5 years and a predominance of male patients (60.9%-88.9%). The number of patients who developed CSCC fluctuated between 10-62, with an average time elapsed since transplant of 5-8 years.
Contrary to the inverted ratio 4:1 of BCC and CSCC described in several anglo-saxonic publications,8 in the Portuguese series this inversion has not been verified.15-18
Instead, ratios of 1:1 have been found between BCC and CSCC. This is similar to other Southern European reports, such as those from Spain19,20and Italy.21 These ratios are attributed to genetic differences as well as sun exposure habits.16,18
Table 1 Summary of Portuguese revisions about CSCC in SOTR, in the last 10 years
| Publications | Operation year | Number of transplant recipients | Mean age+ | Male gender | Number of patients that developed CSCC | Time interval from transplant until CSCC* | Ratio BCC: CSCC |
|---|---|---|---|---|---|---|---|
| Fernandes et al15 | 2000-2010 | 319 (180 renal and 230 hepatic) | 60.5 - renal 63.5 - hepatic | 83.3% - renal 88.9% - hepatic | 18 | 55.2 - renal 36 - hepatic | 1:1.3 - renal 3.5:1- hepatic |
| Borges-Costa et al16 | 2010-2011 | 127 renal | 53 | 67% | 10 | 96 | 1:1 |
| Pinho et al17 | 2004-2013 | 288 renal | 61.9 | 66% | 62 | 64 | 1:1 |
| Garrido et al18 | 2008-2014 | 197 renal | 50.7 | 60.9% | 15 | Not specified | 1.1:1 |
RISK FACTORS
The risk of developing a CSCC at 10 years post-transplant is estimated to be 10%-27% and at 20 years post-transplant is 40%-60%.9
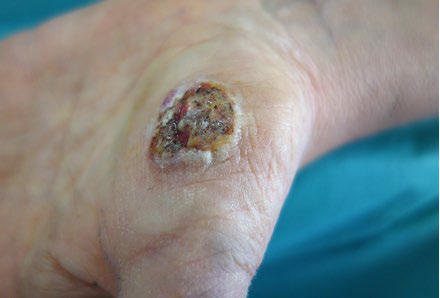
The most contributing factor is ultraviolet (UV) radiation, explaining why the majority of CSCC occur in sun-exposed locations (Fig.s 1 and 2).22Additional contributing factors are advanced age, male gender, skin phototype I or II, infection with beta human papillomaviruses (HPV), genetic factors and the immunosuppressive medication that is needed to maintain the transplanted organ.16,17,23
UV radiation exposure causes mutations in tumor suppressor genes, such as TP53 and NOTCH, and oncogenes, like HRAS, NRAS and KRAS.24 Even sun-exposed skin, without clinical abnormalities, has a similar amount of mutations when compared with oropharyngeal squamous cell carcinomas, although it has a 10-fold lower mutations amount than that reported for CSCC.24
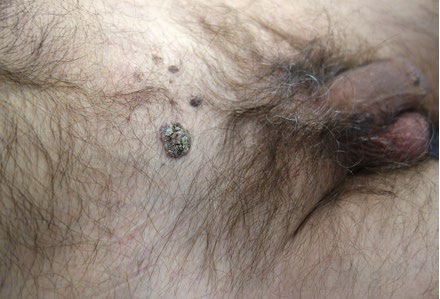
HPV have also been implicated in the pathogenesis of CSCC (Fig. 3).25,26It is well established the role of alpha-genus HPV, such as HPV 16 and HPV 18, in the development of anogenital carcinoma, and they are also involved in the formation of head and neck CSCC and periungual CSCC.27
Beta-genus HPV, like HPV5, HPV8 and HPV9 can be present in the skin of healthy people, but their levels rise with immunosuppression.28,29Their presence has been found in almost 90% of CSCC in SOTR and has been associated with twice the risk of CSCC in SOTR.28,29
A supporting finding for the implication of HPV in CSCC is that actinic keratosis, which are precursors of CSCC, also have an elevated number of Beta-HPV copies.30
The mechanism of action that is hypothesized in the genesis of anogenital CSCC involves the inhibition of TP53 and retinoblastoma protein by the HPV’s oncoproteins E6 and E7.31 Beta-HPV may allow the accumulation of UVR induced mutations, through several mechanisms, for instance, degradation of Bak (involved in cellular apoptosis) and inhibition of Notch tumor suppression.24,27,28
Regardless of these findings, there are some studies in which HPV is found in low or absent activity in CSCC, and this led to the “hit-and-run” hypothesis, that states that Beta-HPV are involved in the formation of the tumor but they are not necessary for its maintenance, contrary to alfa-HPV in anogenital SCC.27,28,31
Another important risk factor is the immunosuppressive medication used for the organ maintenance.32 Sirolimus has been demonstrated to reduce the number of CSCC in comparison to calcineurin inhibitors tacrolimus and cyclosporine.33 The benefit of switching to sirolimus is supposed to be greatest after the development of one CSCC and negligible when there are multiple prior CSCC. Therefore, it has been suggested to switch from calcineurin inhibitors to sirolimus after the first post-transplant CSCC.3,8,13Despite this, sirolimus has more serious adverse events per patient, prompting more patients to discontinue treatment, and in a meta-analysis it has been associated with an increased risk of death, although this remains controversial.33,34
Azathioprine, that acts as a photosensitizer to UVA, is also responsible for an increased risk of CSCC.16Mycophenolate mofetil seems to be associated with lesser risk and should be considered as an alternative option to azathioprine.35
All the factors cited above, act synergistically and as a result of an interaction there can be development and progression of CSCC in solid organ transplant recipients.24
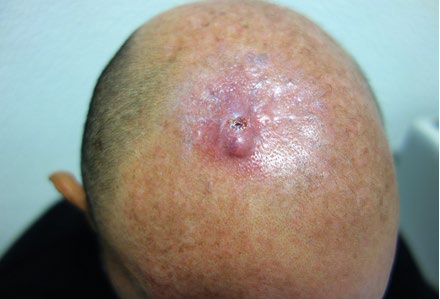
Figure 2 Recurrence of cutaneous squamous cell carcinoma on the head of a renal transplant recipient. Squamous cell carcinoma is more frequent in sun-exposed locations.
PREVENTION
Since SOTR have a higher incidence of CSCC and these are more aggressive, with an increased risk of metastasis of 5%-7%, skin cancer prevention is essential.36
Given that the most important risk factor is UV radiation, emphasis on sunscreen use and sun protection must be made.9
Before transplant, patients should have a full-body skin examination, with a subsequent periodic assessment depending on previous or concomitant skin premalignant and malignant lesions.9 (Table 2). An annual screening is suggested for patients without pre-malignant or malignant lesions. Patients with actinic keratosis or one prior nonmelanoma skin cancer should be evaluated at 3-6 months and if a high-risk CSCC is found the follow-up should be every 3 months. In patients presenting with a metastatic squamous cell carcinoma, an examination after 1-3 months is advised.9
Table 2 Recommended screening interval, according to patient’s risk factors
| Risk factors | Periodic assessment |
|---|---|
| Absence of pre-malignant / malignant lesions | Annual screening |
| Pre-malignant lesions/ One prior nonmelanoma skin cancer | 3-6 months |
| Multiple prior nonmelanoma skin cancers/ High-risk CSCC | 3 months |
| Metastatic CSCC | 1-3 months |
STAGING
There are three main staging systems used for staging CSCC: the American Joint Committee on Cancer (AJCC), the Union for International Cancer Control (UICC) and, more recently, the Brigham and Women’s Hospital Tumor staging system.13.22These staging systems have into account factors such as: tumor dimensions, perineural invasion, depth of invasion and poorly differentiated histology.13.22
Nevertheless, some criteria with prognostic influence, like tumor location and immunosuppression are not considered in any of the main staging systems, causing a potential underestimation of CSCC prognosis in SOTR.37
TREATMENT
Considering the aggressiveness of CSCC in SOTR, treatment of pre-malignant lesions should be sought out and if there is suspicion of transformation into malignant lesions, biopsy or excision should be performed.38
In terms of topical therapies for the treatment of actinic keratosis, there is a wide range of options available: diclofenac, 5-fluorouracil, imiquimod, laser, cryotherapy, among others.22,38
In single lesions, local destructive options, such as cryotherapy or electrocautery and curettage can be considered.
Clearance of 41% of lesions has been achieved with topical diclofenac and 62% clearance has been obtained with topical imiquimod.39,40Imiquimod is an immunomodulatory agent that has anti-viral and anti-tumor properties.41 It has been advised to limit its use to an area of 60-100 cm2, because albeit rare, imiquimod has been linked to induction of systemic immunostimulation.42 However some studies have demonstrated that the actual systemic absorption of topical imiquimod is minimal and hundreds of patients have been treated without graft rejection.42 For areas with extensive lesions, photodynamic therapy should be considered, and has been proven to be more effective than imiquimod.38,43
For CSCC, surgery is the gold standard treatment, not only because it allows tumor removal but also histologic verification.44 The recommended surgical margins depend on the size of the tumor, with margins of 0.4 cm being advocated for tumors inferior to 2 cm, and margins of 0.6 cm for tumors larger than 2 cm.45
For high risk locations (scalp, ears, eyelids, nose), Mohs micrographic surgery is recommended, but if this is not possible and standard excision is performed, avoidance of significant tissue rearrangement is necessary, before confirming histologic margins are free of tumor.44-46Although radiotherapy for CSCC has declined, it can be a viable alternative when surgical options are not possible, particularly for primary tumors.44 An overall control rate was achieved for 89% of previously untreated CSCC and 68% for ecurrent CSCC.47 Radiotherapy is also used as an adjuvant treatment of surgery, for CSCC with perineural invasion, although it has not been established that it improves survival.48,49
In locally advanced or metastatic CSCC, if surgery or radiotherapy are not feasible, systemic therapy with cytotoxic agents (platinum, interferon-alpha, 5-fluouracil and taxanes), epidermal growth factor receptor (EGFR) inhibitors and immunotherapy can be used.22 However, in SOTR there are few studies evaluating the safety and efficacy of these treatments.22
Depending on the treatment combination used, response rates with chemotherapy have ranged from 14% to 86%, with cisplatin and 5-fluouracil obtaining the best results.50
Complete remission was achieved in 43% of patients treated in one series, however side effects of these treatments should be considered.50
The EGFR inhibitors, cetuximab and panitumumab have shown some response against advanced CSCC, with response rates of 27%, and 31%, respectively, evaluated in immunocompetent individuals.49-52These can represent valid options for tumors expressing high levels of EGFR, however in SOTR there are few studies available.50,51
Immunotherapy with anti-PD-1 monoclonal antibody, specifically cemiplimab, was approved in 2018 for the treatment of advanced CSCC.53 The response rate was 47% with an overall survival of 81%.53 Despite these high response rates, in SOTR, there is a risk of organ rejection with checkpoint inhibitors, that is almost 50% within a month of therapy.22
CHEMOPROPHYLAXIS
For patients who develop more than 5 CSCC/per year, systemic retinoid therapy should be considered.54 Acitretin, a systemic retinoid, has reduced the incidence of actinic keratosis and CSCC in SOTR.55 Starting with a low dose of 10mg/daily is recommended, with maximum doses of 30 mg/ daily, in order to prevent patient loss of compliance, due to adverse events.56
However, there are several contraindications for their use, like pregnancy, severe hyperlipidemia, and a rebound effect when therapy is discontinued.57
For this reason, therapy with systemic retinoids should be carefully weighted, as patients can experience a high number of CSCC in a short period of time.55,56Another option that has been studied for chemoprophylaxis, is oral capecitabine, a pro-drug of 5-fluorouracil, that has also shown to reduce the incidence of pre-malignant and malignant lesions after solid organ transplant, with less side effects than systemic retinoids.58
CONCLUSION
Cutaneous squamous cell carcinoma incidence has been rising and is predicted to continue to increase. These tumors are even more common and aggressive in solid organ transplant recipient patients. Ultraviolet radiation is the most important risk factor for the development of CSCC and so primary prevention must be implemented amongst patients. Immunosuppressive medication is necessary to prevent organ rejection but is also associated with an increased risk of developing skin malignancies. Particularly, old immunosuppressive drugs, such as, azathioprine, cyclosporine and tacrolimus have been linked to a higher incidence of CSCC.
Primary treatment option is surgical excision with surgical margins depending on tumor’s size. Radiotherapy can also be used as a therapeutic alternative. Chemoprophylaxis with acitretin should be considered for patients that develop more than 5 CSCC/per year.