Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Portuguesa de Medicina Geral e Familiar
Print version ISSN 2182-5173
Rev Port Med Geral Fam vol.34 no.3 Lisboa June 2018
ESTUDOS ORIGINAIS
Prevalence of stage 3-5 chronic kidney disease in diabetic patients aged 60 or over
Prevalência de doença renal crónica estádio 3-5 na população diabética tipo 2 com 60 ou mais anos de idade
Teresa Luísa Velosa,1 Ana Maria Santos Pereira Paiva,2 Diana Maria Vieira Costa Ferreira,1 Maria Gorete Neves da Costa,1 Celsa Irene Maciel Ferros,3 Idalina Maria Faria Costa Ferreira3
1. Médica de Medicina Geral e Familiar. USF Lígios, ACeS do Cavado III.
2. Consultora de Nefrologia do Hospital Pedro Hispano. Serviço de Nefrologia, IPO do Porto.
3. Enfermeira. USF Lígios, ACeS do Cávado III.
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Objectives: To estimate the prevalence of stage 3-5 chronic kidney disease (CKD3-5) in elderly patients with type 2 diabetes mellitus (T2DM).
Study design: Quantitative, observational, and descriptive with an analytical component.
Setting: The study was conducted at Unidade de Saúde Familiar (USF) Lígios, between June 1, 2013 and June 31, 2014.
Participants: Patients with T2DM aged 60 or over, monitored at USF Lígios.
Methods: Healthcare professionals completed questionnaires with patients' data: gender, age, height, weight, body mass index, schooling, alcohol consumption, smoking habits, blood pressure, nephrotoxic medication, creatinine, estimated glomerular filtration rate (eGFR) (using the modification of diet in renal disease [MDRD] formula), urea, albuminuria, and haematuria.
Results: In this sample, the prevalence of CKD3-5 in the T2DM aged 60 or over was 15%. The prevalence of CKD3-5 increased with age (60-74 years: 10.5%; 75-84 years: 19.4%; ≥85 years: 39.1%, p<0.01), and was positively associated with illiteracy (eGFR ≥60ml/min/1.73m2: 11.6%; eGFR <60ml/min/1.73m2: 21.2%, p<0.05), and negatively associated with alcohol consumption (p<0.05). The prevalence of persistent albuminuria in the sample was 16.8%. There was a statistically significant relationship between smoking habits and persistent albuminuria (albumin/creatinine ratio [ACR] <30mg/g: 3.5%; ACR ≥30mg/g: 10.3%, p<0.05).
Conclusion: The prevalence of CKD3-5 in this sample of T2DM patients aged 60 or over was 15.0%, and the prevalence of persistent albuminuria was 16.8%.
Keywords: Elderly; Type 2 diabetes mellitus; Chronic kidney disease; Prevalence; Primary care; Prevention.
RESUMO
Objetivo: Estimar a prevalência de doença renal crónica (DRC) estádio 3-5, na população diabética tipo 2 com 60 ou mais anos de idade.
Tipo de estudo: Estudo transversal, descritivo com componente analítica.
Local: O estudo decorreu na Unidade de Saúde Familiar (USF) Lígios, entre 1 de junho de 2013 e 31 de junho de 2014.
Participantes: Doentes com diabetes mellitus tipo 2 (DMT2), com 60 ou mais anos de idade, seguidos na USF.
Métodos: Os profissionais de saúde preencheram questionários com dados dos utentes: sexo, idade, estatura, peso, índice de massa corporal, escolaridade, consumo de álcool, hábitos tabágicos, tensão arterial, medicação nefrotóxica, creatinina, taxa de filtração glomerular estimada (TFGe) pela equação modification of diet in renal disease (MDRD), ureia, albuminúria e hematúria.
Resultados: A prevalência de DRC estádios 3-5 nos utentes diabéticos tipo 2 com 60 ou mais anos de idade foi de 15% nesta amostra. A prevalência de DRC estádios 3-5 aumentava com a idade (60-74 anos: 10,5%; 75-84 anos: 19,4%; ≥85 anos: 39,1%, p<0,01), estava associada a analfabetismo (TFGe ≥60ml/min/1,73m2: 11,6%; TFGe <60ml/min/1,73m2: 21,2%, p<0,05) e inversamente associada ao consumo de álcool (p<0,05). A prevalência de albuminúria persistente na amostra foi de 16,8%. Verificou-se existir uma relação estatisticamente significativa entre hábitos tabágicos e albuminúria persistente (razão albumina/creatinina [RAC] <30mg/g: 3,5%; RAC ≥30mg/g: 10,3%, p<0,05).
Conclusão: A prevalência de DRC estádios 3-5 na população com DMT2 com 60 ou mais anos de idade foi de 15,0% e a prevalência de albuminúria persistente foi de 16,8%.
Palavras-chave: Idosos; Diabetes mellitus tipo 2; Doença renal crónica; Prevalência; Cuidados de saúde primários; Prevenção.
Introduction
Diabetes is recognized as one of the biggest global health problems of the 21st century1 and is the most common cause of chronic kidney disease (CKD) in the elderly.2 The incidence and prevalence of CKD and end-stage kidney disease (ESKD) increases with age, and results in a considerable social and economic burden as progression to ESKD is associated with a higher number of comorbid conditions, shorter life expectancy, and increased treatment costs.3 Although CKD-related mortality declines with age in diabetic patients,4 and only a small portion of elderly patients will progress to ESKD or die from renal failure, there is a very strong association between the decline in glomerular filtration rate (GFR) and cardiovascular-related deaths.3,5 Particularly, elderly patients with type 2 diabetes mellitus (T2DM) and CKD have an increased absolute mortality risk,4 what emphasizes the importance of CKD in clinical outcomes.6 Early identification of CKD in elderly diabetic patients is critical to prevent disease progression and to reduce the risk of global and cardiovascular morbidity and mortality.7
In 2015, in Portugal, the estimated prevalence of diabetes in the adult population (20-79 years old), was 13.3%;8 however, there were significant differences between males (15.9%) and females (10.9%) and between different age groups.8 In the elderly population (60-79 years old), the prevalence of diabetes in Portugal was up to 27.0%.8 According to the 2017 International Diabetes Federation Atlas Report, based on nationally representative, peer-reviewed literature from the last five years, Portugal was the European country with the high-est estimated prevalence of diabetes (13.9% [10.2-17.2]), and one of the European countries with the highest age-adjusted prevalence of diabetes (9.8% [6.9-13.2]).1 Between 2009 and 2015, the ageing of the Portuguese population was responsible for a 13.5% rise in the prevalence of diabetes.8
Diabetes can cause devastating individual suffering due to health complications and the resulting limitations, but it also represents a considerable economic burden and has an important social impact. In 2017, the International Diabetes Federation estimated that approximately 850 billion USD were spent with diabetic patients aged 18-99 on a global scale.1 In 2014, Portugal spent around 1,300 to 1,550 million Euro on costs directly related to diabetes (i.e. diabetes medication, blood glucose test strips and hospitalizations), which represented around 0.7 to 0.9% of its total gross domestic product.8
The increased risk of death in diabetic patients has been previously reported to decrease with increased age.4,9 However, the absolute risk of death among elderly patients is higher when renal complications are also present (microalbuminuria, macroalbuminuria and ESKD), emphasizing the importance of early detection and prevention of renal complications.4 Microalbuminuria is associated with a two to four-fold increase on cardiovascular risk.10 In the elderly, microalbuminuria is associated with age, inflammatory markers and systolic blood pressure (SBP), which may explain the association between microalbuminuria and coronary heart disease.11
In Portugal, in 2015, diabetes accounted for 4% of cases of all deaths; when considering hospital mortality rates only, diabetes was either the main or a secondary cause of death in 2.9% and 9.2% of patients aged 70 or over, respectively.8
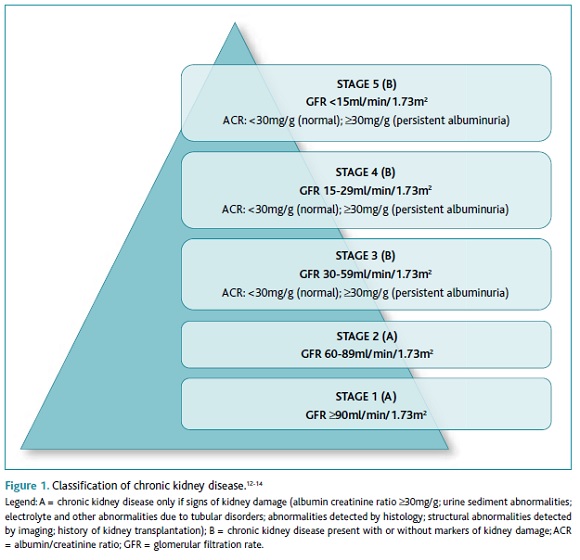
According to the National Kidney Foundation, “CKD is defined as abnormalities of kidney structure or function, present for 3 months or longer, with implications for health”,12 and is an increasing public health issue.7 Stages 3-5 are defined by the presence of an estimated glomerular filtration rate (eGFR) lower than 60ml/min/1.73m2, with or without markers of kidney damage, on at least two occasions, separated by a period of at least 90 days.12-13 Stages 3-5 CKD (CKD3-5) can be classified according to its cause, eGFR value, and albumin/creatinine ratio value (ACR) (Figure 1).12-14 ACR values are classified as normal (<30mg/g), moderately increased or microalbuminuria (30-299mg/g), and severely increased or macroalbuminuria (≥300mg/g).12,14 When remission of albuminuria does not occur within 90 days, patients are diagnosed with persistent albuminuria.12,14 Microalbuminuria is the earliest sign of diabetic kidney disease (DKD).15
A decline in eGFR of about 1ml/min/year after the fourth decade of life is regarded as part of ‘normal ageing'.3 Only a small percentage of elderly patients with compromised renal function will progress or die from renal failure.3 However, incidence and prevalence of ESKD is rising, especially in the oldest groups, most likely reflecting both population ageing and the high overall prevalence of CKD in the elderly.3 The prevalence of CKD is estimated to be 8-16% worldwide.7
Prevalence of CKD3-5 in Portugal was 6.1%.16 However, Portugal has the 8th highest annual incidence of ESKD and the 4th highest prevalence rate of ESKD worldwide.7 According to recent data from the Ministry of Health, based on data from the Integrated Management of Chronic Kidney Disease platform, 36% of the patients on haemodialysis in Portugal were older than 74 years, and high haemodialysis treatment rates were observed in Centro and Alentejo (43 and 46% respectively).17 Amongst haemodialysis patients, 84% of deaths occurred in patients with more than 65 years.17
CKD complications include “increased all-cause and cardiovascular mortality, kidney disease progression, acute kidney injury, cognitive decline, anaemia, mineral and bone disorders, and fractures”.7 As CKD is associated with a higher increase on mortality rates in the elderly, when compared to other age groups,2 and as expenditure with ESKD patients rises abruptly with age, preventing progression of CKD should be an urgent priority, particularly in the oldest patients.3
CKD awareness is low amongst patients and healthcare providers.7 On the other hand, elderly patients pose particular therapeutic challenges to healthcare providers, due to altered pharmacokinetics, co-morbidities, polypharmacy, confusion about taking prescription medications, possible economic limitations, higher risk of drug-induced hypoglycaemia, higher risk of hypotension and adverse events related to the use of hypertensive drugs.2,12 Family physicians must be involved in the early identification, treatment and appropriate referral of patients with CKD.7
The aim of this study was to determine the prevalence of CKD3-5 in elderly patients with T2DM, in a primary care setting.
Methods
Study design
Quantitative, observational, descriptive with an analytical component.
Setting
The study was conducted in a primary health care unit, USF Lígios, part of Agrupamento de Centros Saúde (ACeS) do Cávado III – Barcelos/Esposende, located in the North of Portugal, from June 1, 2013 to June 31, 2014.
Population and sample
T2DM patients aged 60 or over and monitored at USF Lígios were included. Patients were identified by the presence of the code T90 diabetes non-insulin dependent (ICPC-2 – International Classification of Primary Care, 2nd ed.) in their electronic health file. Exclusion criteria included patients with unstable creatinine values (i.e. defined as a change within a 40-week period in eGFR values of 25% or more in the absence of acute renal injury),18 patients with extreme muscle mass values (i.e. patients who have had an amputation, with malnutrition or muscle-wasting conditions, including the frailty syndrome),15 and home-care patients. Frailty syndrome was defined as the presence of three or more of the following criteria: shrinkage (weight loss), self-reported exhaustion, weaknesses, low gait speed and low physical activity.19 Progressive loss of muscle mass and strength or sarcopenia is a key component of frailty syndrome, leading to functional impairment and physical disability. Sarcopenia results from physiologic changes related to the ageing process, which include anorexia, or from severe disease.19-20 A probabilistic systematic sample was obtained, as all T2DM patients were convened to participate in the study, and in case of default were rescheduled.
Informed consent
Patients involved in the study gave written informed consent. The investigators provided a simple and concise explanation of the scope and objectives of the study to ensure that patients understood what it entailed. Anonymity was guaranteed to participants, which were also informed of the possibility of withdrawing from the study at any time.
Data collection
Family nurses filled in anthropometric and blood pressure (BP) data on the questionnaires, which were then handed out to the family physician to fill in remaining data. The questionnaires were tagged for identification.
Collected variables included: gender, age, education, median of two height measures and one weight measure (following the Third National Health and Nutrition Examination Survey [NHANES III] anthropometric measurement procedures, and using a previously calibrated Digital Weighing & Measurement Station Seca 220®), body mass index (BMI) (categorized according to the World Health Organization classification [i.e. underweight: <18; normal: 18-24; overweight: 25-29; obese class I: 30-34; obese class II: 35-39; obese class III: ≥40]), alcohol consumption (grams per week), smoking habits (none, passive, and active as packs per year), average SBP and diastolic blood pressure (DBP) (measured in the left arm, three readings, interval between readings of thirty seconds, using Omron M5 Professional®), use of nephrotoxic medication (i.e. nonsteroidal anti-inflammatory drugs [NSAID], ciclo-oxigenase-2 [COX 2] inhibitors, lithium, angiotensin receptor blockers [ARB] and angiotensin-converting enzyme [ACE] inhibitors if bilateral renal artery stenosis or unilateral in single kidney, among others), creatinine (one measurement if eGFR ≥60ml/min/1.73m2 or two measurements within a period of at least 90 days if eGFR <60ml/min/1.73m2; eGFR was calculated using the modification of diet in renal disease [MDRD] formula), ACR (one measurement if <30mg/g or two measurements within a period of at least 90 days if ≥30mg/g), and haematuria (assessed by urine test strip, and confirmed by a second reading if positive). Second creatinine and ACR measurements were performed to confirm, or not, the presence of CKD3-5 and persistent albuminuria. Mean values of two measurements of creatinine, eGFR and ACR were used for data analysis (Table 1 and Table 2).
The variables age, height, weight and ACR were rounded to one decimal place. Creatinine and MDRD eGFR were rounded to two decimal places.
Variables were categorized as follows: age (60-74 years, 75-85 years, ≥85 years); BMI (normal and overweight/obese); education (illiterate and ≥ 1 years); alcohol consumption (<70g/week or >70g/week);21 smoking status (smoker, which included passive smoking or non-smoker); BP (<130/80mmHg or ≥130/80mmHg); use of nephrotoxic medication (present or absent).
Ethical aspects
Confidentiality was ensured as questionnaires were tagged with a code starting with the first letter of each family physician's name and the number of the patient by input order in the study (ex: A-1; A-2; etc.). Another record sheet was filled in by each family physician, which matched all the participants' study code numbers with their operating numbers in order to insert the results of other investigations during the study. This record sheet also included the motives for patient exclusion or drop outs and was destroyed six months after the end of the collecting data period.
Data collection was authorized by the National Data Protection Committee, Proc. 648/2013, Authorization 983/2013, on February 5, 2013. The study protocol was approved by the Ethics Health Committee of the North Regional Health Administration, Approval 20/2013, on March 23, 2013, which included the authorization of both the ACeS and the USF boards.
Data analysis
Kolmogorov-Smirnov test was used to test the normality of the data distribution;22 Chi-squared test was used to test differences between sets of categorical data;23 Mann-Whitney U test was used to compare differences between two independent groups when the dependent variable was not normally distributed.24 Results were expressed either as median (plus minimum and maximum) or as frequency distribution, as appropriate. A significance threshold of p<0.05 was adopted. Data were compiled using Microsoft Office Excel 2007® and SPSS version 22.0® (IBM SPSS statistics) was used for statistical analysis.
Results
Sample characteristics
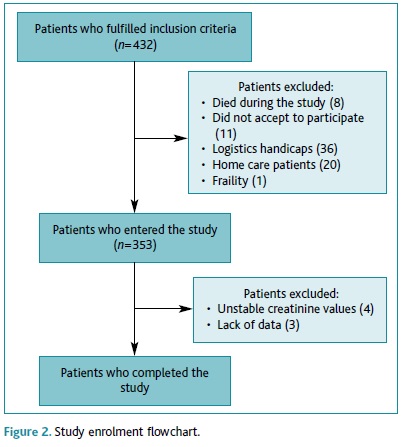
At the time the study was conducted, USF Lígios had 432 diabetic patients aged 60 or over, of whom 346 (80.1%) enrolled this study. Eighty-six patients did not participate in this study: eight (1.9%) died during the study, 11 (2.5%) declined to participate, 36 (8.3%) didn't participate due to feasibility reasons (lack of time during consultations), 20 (4.6%) were home-care patients, three (0.7%) had undergone an amputation and one had frailty syndrome (0.2%). We further excluded four (0.9%) patients because of unstable creatinine values and three (0.7%) patients due to incomplete data (Figure 2).
Participant characteristics
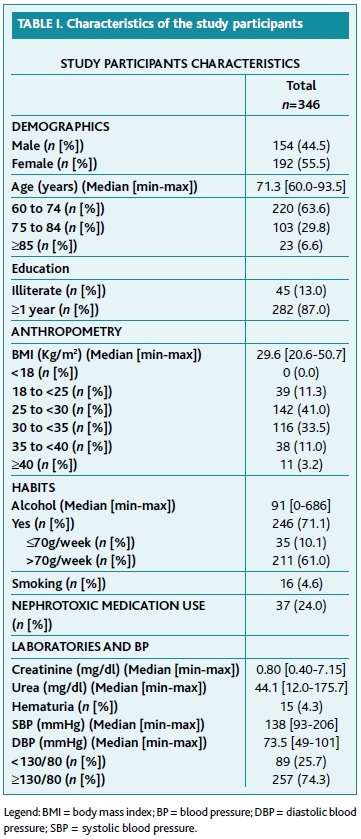
Table 1 describes demographic and anthropometric characteristics, as well as habits, use of nephrotoxic medication, BP values and biochemical measurements of the participants included. The total sample comprised 346 patients, with a median age of 71.3 years [average: 60.0-93.5]. Female to male ratio was 1.27:1. Most of patients had less than four years' education (81.5%) and 13.0% were illiterate. The median BMI was 29.6 [20.6-50.7] kg/m2 and most of participants were obese (47.7%) or overweight (41.0%). Heavy drinking habits were observed in both genders, with a median value of 91 [0-686] g/week. Most of patients did not report passive exposure to cigarette smoke nor active smoking (95.4%). About 26% of patients used nephrotoxic medication (19.4% NSAID, 6.1% COX 2 inhibitors, 0.6% both, and 0.3% tacrolimus). Median creatinine value was 0.80 [0.40-7.15] mg/dl, and median urea value was 44.1 [12.0-175.7] mg/dl. Haematuria was present in 4.3% of participants. Median SBP was 138 [93-206] mmHg, and median DBP was 73.5 [49-101] mmHg.
Non-participant characteristics
Non-participants had a mean age of 75.8 [SD=9.4], with a female to male ratio of 1.97:1. This group included those with decreased muscle mass (homecare patients, previous amputation, and frailty).
Prevalence of CKD3-5 and of persistent albuminuria
Sixty-one diabetic patients had MDRD eGFR below 60ml/min/1.73m2 in a first creatinine measurement, of whom 52 (85.2%) were confirmed to have CKD3-5. Also, 69 diabetic patients had ACR ≥30mg/g at first measurement, of whom 58 (79.7%) were confirmed to have persistent albuminuria.
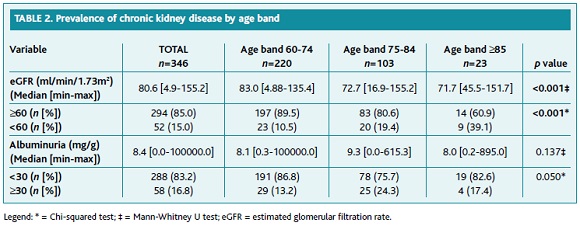
Table 2 describes the prevalence of CKD and persistent albuminuria. The prevalence of CKD3-5 in the sample was 15.0%, and a statistically significant difference was observed between age groups (60-74 years: 10.5%; 75-84 years: 19.4%; ≥85 years: 39.1%, p<0.01).
The prevalence of persistent albuminuria was 16.8%. No statistically significant relationship was found between age groups and persistent albuminuria.
Relationship between CKD and patients' characteristics
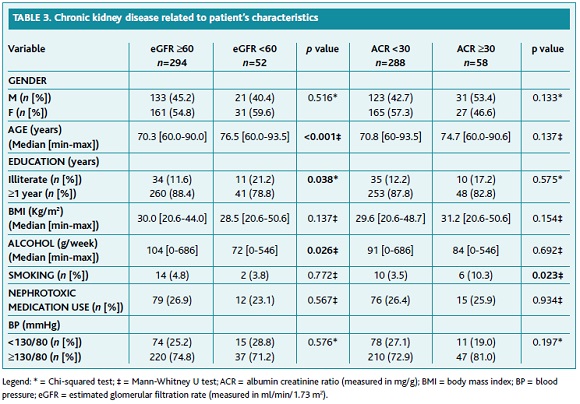
Table 3 describes the relationship between CKD and patients' characteristics. In multivariate analysis, no significant differences were found between gender both for the presence of CKD and of persistent albuminuria. BP values were below 130/80mmHg in 25.7% of patients. No statistically significant relationship was found between BP and CKD, or with persistent albuminuria.
A statistically significant relationship was found between education and the presence of CKD3-5, with illiterate patients exhibiting a higher prevalence (eGFR ≥60ml/min/1.73m2: 11.6%; eGFR <60ml/min/1.73m2: 21.2%, p<0.05). Conversely, a negative relationship was observed between alcohol consumption and CKD3-5 (p<0.05).
Smoking habits were significantly related to persistent albuminuria (ACR <30mg/g: 3.5%; ACR ≥30mg/g: 10.3%, p<0.05), but not to CKD.
No significantly statistical relationships were found between BMI, alcohol intake, the use of nephrotoxic medication and kidney damage.
Discussion
In this study, the prevalence of CKD3-5 in T2DM patients aged 60 or over was 15.0%, and the prevalence of persistent albuminuria was 16.8%.
A study carried out in the United States (U.S.) from 1999 to 2012, based on the National Health and Nutrition Examination Survey (NHANES), found that the prevalence of CKD3-5 and albuminuria in T2DM patients aged 65 or over was, respectively, 43.1% and 39.1%.25 A more recent study estimated the prevalence of CKD3-5 from 13 European countries, including Portugal, in the adult population, stratifying by age, gender and risk factors such as diabetes.26 In T2DM patients, studies using non Isotope Dilution Mass Spectrometry (IDMS) traceable creatinine, the prevalence of CKD3-5 in the age group between 65-74 years old varied between 16.2% (4.3-28.1) in Italy, and 30.7% (21.7-39.7) in Portugal, whereas in the age group between 75-84 years old, the prevalence of CKD3-5 varied between 34.8 (29.6-40.0) in Poland, 52.3% (41.8-62.9) in Portugal, and 66.0% (62.8-69.2) in the United Kingdom.26 The European study identified a substantial variation between countries, and within countries, that appeared to be due to factors other than the prevalence of diabetes, hypertension or obesity. In our study, prevalence of CKD3-5 was lower than those reported from the U.S. and Europe.
Prevalence estimates are influenced using different creatinine and albuminuria measurement methods, different equations to estimate CKD, and specific study methodologies. In the present study, MDRD was used as it is a reliable tool to estimate eGFR <60ml/min/1.73m2.27 MDRD has not been validated in patients older than 70 years of age, but the National Institute of Diabetes and Digestive and Kidney Diseases considers its use in older people.28 eGFR estimates based on serum creatinine are less accurate for patients at the extremes of muscle mass, such as the frail elderly and the critically ill,15 so we excluded these patients, which might have contributed to an underestimation of the prevalence of CKD3-5. We also did not rely on a single assessment, as we confirmed the values if eGFR <60ml/min/1.73m2, and if albuminuria ≥30mg/g with at least 90 day's intervals. The sample of this study, predominantly composed by non-differentiated workers from a rural setting, had some hallmark characteristics: high illiteracy rate (13.0%), low educational level (81.5%), and heavy alcoholic use (61.0%). Unknown or regional factors could have contributed towards the observed results, as prevalence of haemodialysis is higher in the South and Centre of Portugal.17
According to the National Institute for Health and Care Excellence guidelines (2014), which recommend a SBP below 130mmHg and a DBP below 80mmHg in people with CKD and diabetes,14 BP was controlled in 25.7% of cases (Table 3). In a similar study carried out in two Netherlands Primary Health Care Centres, in 2006,29 the prevalence of CKD was 28.0% amongst T2DM adults, and mean SBP and DBP were, respectively, 138±18mmHg and 81±8mmHg. In the present study, median SBP and DBP values were comparable (138 [93-206] and 73.5 [49-101], respectively).
As expected, CKD3-5 significantly increased with age,3 also increasing the risk of cardiovascular disease.30 Preventive measures are warranted in these patients to slow the progression of kidney disease, such as the use of antihypertensive medication, particularly ACE inhibitors and ARB, adequate BP control, moderate-protein diets, and intensive management of blood glucose, and to provide cardiovascular protection through healthy lifestyle habits, and antiaggregant and lipid-lowering medication.
In this sample, a significant association was found between illiteracy and the presence of CKD3-5. We haven't found any study specifically referring illiteracy to be linked with CKD. However, several studies have shown a link between low literacy levels and higher risk of CKD when compared to those with tertiary education.31-35 Differences in behavioural patterns (i.e. poor diet, smoking, and obesity), occupational and environmental exposures, associated comorbid conditions and access to healthcare, could explain the higher risk of CKD in those with low educational levels.36-37 Regarding illiteracy in CKD patients, it has been reported to be associated with higher risk of vascular events and all-cause mortality, although this link was almost eliminated after adjustment for smoking, obesity, alcohol consumption and comorbid conditions.37
In this study, the smoking was found to be significantly related to persistent albuminuria. Interestingly, a recent study in Korea38 compared the effects of tobacco smoke before and after the diagnosis of diabetes and the risk of diabetic nephropathy. The definition of diabetic nephropathy used in the Korean study included the presence of albuminuria (spot urine ACR ≥30mg/g) or low estimated glomerular filtration rate (<60mL/min/1.73m2). In that study, male patients who continued smoking after the diagnosis of diabetes had higher outcomes of diabetic nephropathy compared with those who quit smoking after the diagnosis of diabetes and with non-smokers. The authors concluded that tobacco use in patients with a known diagnosis of diabetes was positively associated with CKD and strengthened the importance of smoking cessation programs.
Finally, a negative association was found between alcohol intake and the prevalence of CKD3-5. The National Kidney Foundation (NKF) reports the benefits of limiting alcohol intake to BP and health in general and therefore they adopted the same recommendations to CKD patients.39 However, this is not the first study to observe an inverse association between alcohol consumption and CKD.40-41 Indeed, a prospective study accomplished by the International Society of Nephrology found a negative association between alcohol consumption and the risk of developing CKD when defined as an eGFR <60ml/min/1.73m2 or an ACR >30mg/g.42 Nevertheless, alcohol intake should not be perceived as protective towards CKD. Alcohol intake is a risk factor for poor treatment adherence, hypoglycaemia when concomitantly using insulin or sulfonylurea, obesity, gastrointestinal diseases, neuropsychiatric disorders, liver cirrhosis, glomerulonephritis, disruption of water and electrolyte balance, acute kidney injury, alterations in body's acid-base balance, kidney graft failure, malnutrition, alcoholic cardiomyopathy, oral cancer and pharynx cancer, pancreatitis, laryngeal cancer, oesophageal cancer, interpersonal violence, self-harm, poisoning, drowning, falls, liver cancer, epilepsy, haemorrhagic stroke, ischaemic heart disease, tuberculosis, among other detrimental health effects.43-44 Therefore, alcohol is ought to be consumed according to NKF recommendations.
Strengths and limitations
To the best of our knowledge, this is the first study that estimates the prevalence of CKD3-5 in elderly T2DM patients, in Portugal. A strength of our study was the accuracy of the measurements, as BP was taken as the mean value of three repeated measurements, and the creatinine, ACR values and persistent albuminuria were confirmed with at least 90 days' intervals, accomplishing international definitions of CKD. Other strengths of the study were the wide range of variables incorporated and clear inclusion and exclusion criteria. We excluded home-care patients who are usually critically ill, patients with unstable creatinine values and patients at the extremes of muscle mass, according to NKF recommendations,15 as these are sources of error when estimating eGFR using creatinine.12
One of the limitations of the study is its cross-sectional nature, as we did not aim to explore etiologic aspects, rate of progression of CKD and of albuminuria, and cardiovascular risk stratification. Furthermore, no power size calculations were performed. This study comprised a relatively small sample, representing a particular setting, and nationwide studies, encompassing several centres, should be undertaken to provide a more accurate estimate of the prevalence of CKD3-5 and of persistent albuminuria, in this subset of patients.
Conclusion
As diabetes is the main risk factor for CKD, and as CKD significantly reduces life expectancy, is critical to estimate the prevalence of CKD to inform CKD management and prevention policies. The prevalence of CKD in T2DM patients aged 60 or over was 15% in this sample and increased significantly with age. Cardiovascular preventive procedures ought to be adopted in these patients, focusing on population with high illiteracy rates, and incorporating recommendations on smoking cessation, healthy lifestyle habits and pharmacological approaches.
REFERENCES
1. International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: IDF; 2017. [ Links ]
2. Williams ME, Stanton RC. Kidney disease in elderly diabetic patients. In: American Society of Nephrology, editor. Geriatrics nephrology curriculum. Washington: ASN; 2009. chap. 8. [ Links ]
3. Wiggins J, Patel S. The coming pandemic of CKD/ESKD and the aging population. In: American Society of Nephrology, editor. Geriatrics nephrology curriculum. Washington: ASN; 2009. chap. 3. [ Links ]
4. Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720-32. [ Links ]
5. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. Inflammation and cardiovascular events in individuals with and without chronic kidney disease. Kidney Int. 2008;73(12):1406-12. [ Links ]
6. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662-73. [ Links ]
7. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260-72. [ Links ]
8. Sociedade Portuguesa de Diabetologia. Diabetes: factos e números (o ano de 2015) - Relatório anual do Observatório Nacional da Diabetes. Lisboa: SPD; 2016. [ Links ] ISBN 9789899666320
9. Lind M, Garcia-Rodriguez LA, Booth GL, Cea-Soriano L, Shah BR, Ekeroth G, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia. 2013;56(12):2601-8. [ Links ]
10. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635-701. [ Links ]
11. Barzilay JI, Peterson D, Cushman M, Heckbert SR, Cao JJ, Blaum C, et al. The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: the cardiovascular health study. Am J Kidney Dis. 2004;44(1):25-34. [ Links ]
12. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [ Links ]
13. Carville S, Wonderling D, Stevens P, Guideline Development Group. Early identification and management of chronic kidney disease in adults: summary of updated NICE guidance. BMJ. 2014;349:g4507. [ Links ]
14. National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. London: NICE; 2014. [ Links ]
15. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266. [ Links ]
16. Vinhas J, Gardete-Correia L, Boavida JM, Raposo JF, Mesquita A, Fona MC, et al. Prevalence of chronic kidney disease and associated risk factors, and risk of end-stage renal disease: data from the PREVADIAB study. Nephron Clin Pract. 2011;119(1):c35-40.
17. Direção-Geral da Saúde. Tratamento conservador médico da insuficiência renal crónica estádio 5: norma da Direção-Geral da Saúde n.º 017/2011, de 28/09/2011, atualização em 14/06/2012. Lisboa: DGS; 2012. [ Links ]
18. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25 (Pt 3):259-63. [ Links ]
19. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56. [ Links ]
20. Khezrian M, Myint PK, McNeil C, Murray AD. A review of frailty syndrome and its physical, cognitive and emotional domains in the elderly. Geriatrics. 2017;2(4):36. [ Links ]
21. Direção-Geral da Saúde. Deteção precoce e intervenção breve no consumo excessivo de álcool: norma da Direção-Geral da Saúde n.º 30/2012, de 28/12/2012, atualização em 18/12/2014. Lisboa: DGS; 2014. [ Links ]
22. Lilliefors HW. On the Kolmogorov–Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62(318):399-402. [ Links ]
23. Agresti A, Kateri M. Categorical data analysis. In: Lovric M, editor. International encyclopedia of statistical science. New York: Springer; 2011. p. 206-8. ISBN 9783642048982 [ Links ]
24. Hart A. Mann-Whitney test is not just a test of medians: differences in spread can be important. BMJ. 2001;323(7309):391-3. [ Links ]
25. Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [ Links ]
26. Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27(7):2135-47. [ Links ]
27. Munikrishnappa D. Limitations of various formulae and other ways of assessing GFR in the elderly: is there a role for cystatin C? In: American Society of Nephrology, editor. Geriatrics nephrology curriculum. Washington: ASN; 2009. chap. 6. [ Links ]
28. National Institute of Diabetes and Digestive and Kidney Diseases. Estimating glomerular filtration rate [homepage]. Washington: NIDDKD; [s.d.] [2016 Oct 2]. Available from: https://www.niddk.nih.gov/health-information/communication-programs/nkdep/laboratory-evaluation/glomerular-filtration-rate/estimating [ Links ]
29. Van der Meer V, Wielders HP, Grootendorst DC, de Kanter JS, Sijpkens YW, Assendelft WJ, et al. Chronic kidney disease in patients with diabetes mellitus type 2 or hypertension in general practice. Br J Gen Pract. 2010;60(581):884-90. [ Links ]
30. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050-65. [ Links ]
31. Adjei DN, Stronks K, Adu D, Snijder MB, Modesti PA, Peters RJ, et al. Relationship between educational and occupational levels, and chronic kidney disease in a multi-ethnic sample: the HELIUS study. PLoS One. 2017;12(11):e0186460. [ Links ]
32. Choi AI, Weekley CC, Chen SC, Li S, Tamura MK, Norris KC, et al. Association of educational attainment with chronic disease and mortality: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2011;58(2):228-34. [ Links ]
33. Fored CM, Ejerblad E, Fryzek JP, Lambe M, Lindblad P, Nyrén O, et al. Socio-economic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18(1):82–8. [ Links ]
34. Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159(15):1777-83. [ Links ]
35. Henry Ford Health System. Chronic kidney disease (CKD): clinical practice recommendations for primary care physicians and healthcare providers (a collaborative approach). HFHS; 2011. [ Links ]
36. Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff. 2002;21(2):60-76. [ Links ]
37. Morton RL, Schlackow I, Staplin N, Gray A, Cass A, Haynes R, et al. Impact of educational attainment on health outcomes in moderate to severe CKD. Am J Kidney Dis. 2016;67(1):31-9. [ Links ]
38. Yeom H, Lee JH, Kim HC, Suh I. Tobacco smoking after diagnosis of diabetes and the risk of diabetic nephropathy. Circulation. 2016;133 Suppl 1:AP066. [ Links ]
39. National Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337-414. [ Links ]
40. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Brabec BA, O'Corragain OA, Edmonds PJ, et al. High alcohol consumption and the risk of renal damage: a systematic review and meta-analysis. QJM. 2015;108(7):539-48. [ Links ]
41. Hsu YH, Pai HC, Chang YM, Liu WH, Hsu CC. Alcohol consumption is inversely associated with stage 3 chronic kidney disease in middle-aged Taiwanese men. BMC Nephrol. 2013;14:254. [ Links ]
42. Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJ, Joosten MM, et al. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int. 2015;87(5):1009-16. [ Links ]
43. World Health Organization. Global status report on alcohol and health 2011. Geneva: WHO; 2011. ISBN 9789241564151
44. Epstein M. Alcohol's impact on kidney function. Alcohol Health Res World. 1997;21(1):84-92. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Teresa Luísa Velosa
E-mail: teresavelosa@gmail.com
http://orcid.org/0000-0003-4333-4716
Acknowledgements
We thank to all professionals of USF Lígios who kindly contributed to make this work possible: Carlos Monteiro, Conceição Cardoso, Diana Rio, Eduardo Sobral, Fernanda Campos, Joana Sarmento, Laura Fonseca, Rita Pereira, and Sónia Costa.
Conflict of interests
The authors have no other conflicts of interest to declare.
Recebido em 05-10-2016
Aceite para publicação em 02-03-2018