Introduction
Most female pelvic masses result from gynaecological diseases, typically involving the ovaries or the uterus, and less commonly the Fallopian tubes. Non-gynaecological cases encompass tumours arising from the bladder or ureters, the gastrointestinal tract, the peritoneum or extra-peritoneal structures, including vessels and lymph nodes, and finally the surrounding musculoskeletal structures1
While the origin of small tumours may be tale-telling to recognize, it may become daunting in larger pelvic tumours. The first step in a rational approach to female pelvic tumours in computed tomography (CT) or magnetic resonance imaging (MRI) is to identify the normal structures inside the pelvis, the walls of the pelvic cavity, and the various peritoneal folds and recesses. Pelvic tumours frequently displace nearby organs out of their usual location, so the relationship with surrounding structures should be assessed. The recognition of different patterns of displacement is particularly important to determine if the tumour is intra or extra-peritoneal. After that global appreciation, the organ of origin has to be determined. Some organ-specific signs may be used to recognize it.
The authors aim to review the main radiological signs available for determining the origin of female pelvic masses and to develop a rational algorithm to identify the place of origin of undetermined tumours on CT and MRI. The discussion of histological diagnoses is beyond the purpose of this manuscript.
Is it intra or extra-peritoneal?
Determining if a tumour is intra or extra-peritoneal should be the first step in an imaging approach to female pelvic tumours. The radiologist must be familiarized with the normal anatomy of the peritoneal reflections and both intra and extra-peritoneal spaces. Moreover, patterns of displacement should be assessed.1,4
Before explaining the patterns of displacement, we should come back to the basics and clarify some anatomic nomenclature. The ovaries are the only intra-peritoneal gynaecological organs.5 The uterus and Fallopian tubes are extra-peritoneal, but its imaging presentation may resemble that of intra-peritoneal structures particularly in the set of huge tumours. It happens because the body of the uterus and Fallopian tubes are almost entirely covered by peritoneum. On the other hand, the cervix is placed below the broad ligaments and behaves as it is: an extra-peritoneal organ. We should also note that some diseases can be multispatial and transpatial, so this evaluation should be cautious in the case of invasive tumours, pelvic abscesses or haematomas (Fig. 1).3
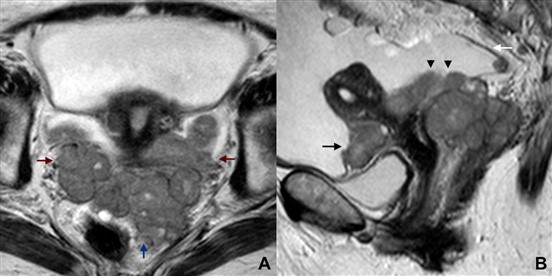
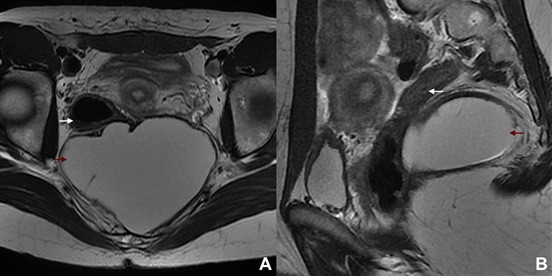
Figure 1: Serous carcinoma of the left ovary in a 61-year-old woman. (A) Axial and (B) sagittal T2W MR images show a huge solid tumour encompassing both extra and intra-peritoneal spaces. The posterior peritoneal reflection is depicted (white arrow), and the tumour may be seen above and below it. Both the vesico-uterine pouch (black arrow) and the cul- de-sac (black arrowheads) are occupied. Inferiorly, both parametria (red arrows) and the meso-rectum (blue arrow) are invaded. Ascites is also found.
Extra-peritoneal tumours
The extra-peritoneal space lies anterior, lateral, posterior, and caudal to the peritoneal reflections, comprising the pelvic sidewall, bladder, uterus, Fallopian tubes, pelvic ureters, iliac vessels, pelvic lymph nodes, and the lower third of the rectum1,4,5
The iliac vessels tend to be effaced, encased, or anterior and medially displaced in the set of an extra-peritoneal tumour(Fig. 2).1 Similarly, the ureters tend to be anterior or medially displaced by extra-peritoneal tumours (Fig. 3).6,7 These are general rules that should not be strictly applied, as some important exceptions may be found. For example, bulky uterine and bladder masses may project into the peritoneal cavity and cause lateral or posterior displacement of the ureters, despite being extra-peritoneal in origin.1
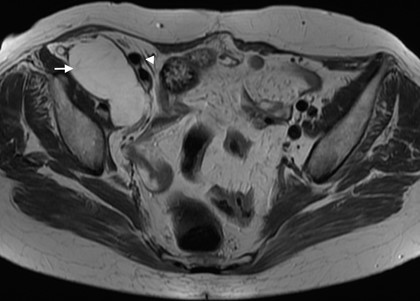
Figure 2: A retro-peritoneal low-grade appendiceal mucinous neoplasm in a 69-year-old woman. T2W MR image shows a cystic, homogeneous mass (arrow) displacing the external iliac vessel medially (arrowhead).
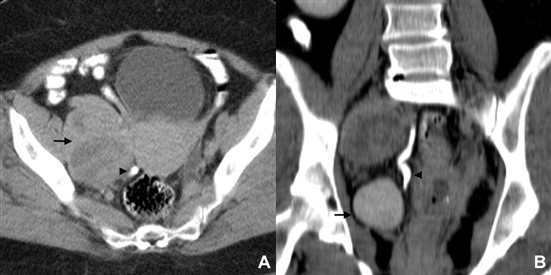
Figure 3: Pelvic lymph node metastasis in a 60-year- old woman with a cervical carcinoma. (A) Axial and (B) coronal CT enhanced images acquired in the excretory phase show a right huge obturator lymph node metastasis (arrow) displacing the ureter medially (arrowhead).
The rectum may be anteriorly and/or laterally displaced by pre-sacral tumours(Fig. 4).3,6 A specific sign may be found in neurogenic pre-sacral tumours: the “ball-on-a string” sign. It is typically seen as a well-defined solid fusiform- shaped mass related to the neurovascular bundle that arises from sacral foramen.8,9 Finally, effacement of the pelvic wall muscles also indicates extra-peritoneal origin of a tumour.1
Intra-peritoneal tumours
The intra-peritoneal space lies superior to the pelvic peritoneal reflections, containing the ovaries, bowel and the upper two-thirds of the rectum.1,5 Intra-peritoneal tumours tend to displace the iliac vessels, body of the uterus and bowel laterally. The ureters may be displaced lateral and posteriorly.3,7 Once again, we should highlight that uterine and tube tumours, which are extra-peritoneal, may grow and behave like intra-peritoneal tumours. Parietal peritoneum covers most of their surface, so that a huge mass may project into the peritoneal cavity and displace the iliac vessels and bowel laterally (Fig. 5), and the ureters posterior and laterally, therefore mimicking an intra- peritoneal lesion.
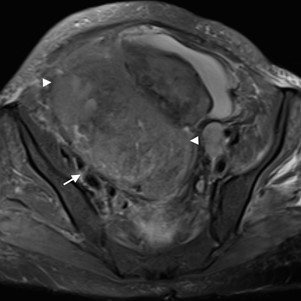
Figure 5: Endometrial carcinoma in a 62-year-old woman. Axial T2W MR image shows a huge endometrial carcinoma (arrowheads) displacing the right iliac vessels laterally (arrow). Despite being extra-peritoneal, this tumour projects into the peritoneal cavity and displaces the surrounding structures like a typical intra-peritoneal tumour.
Is it from the ovary?
The ovarian morphological features greatly vary with age and the hormonal status of the patient. It is also to keep in mind that the triple ovarian anchoring system laxity and inherent variations (the mesovarium, the utero-ovarian ligament and the suspensory ligament) allows a great ovarian mobility, so the position of the ovaries is highly variable in the pelvis, sometimes reaching the lower abdomen.1 In the presence of large ovarian or extra-ovarian pelvic masses the ovaries may undergo considerable displacement, but non-pathological factors like the uterine size and position, degree of filling of the urinary bladder, and degree of distension of the recto-sigmoid colon should also be considered. Moreover, in pregnancy the ovaries are pushed up in the pelvis and after delivery they may or may not return to the initial position.10
The visualization of the ovarian anchoring system on imaging studies becomes easier in the set of ascites (Fig. 6). In the absence of intra-peritoneal fluid, these ligaments may be collapsed against the ovary, the Fallopian tubes and the pelvic sidewall, therefore hampering its identification. The suspensory ligament is a good anatomic clue for ovarian localization. This 4-5 cm long fan-shaped band of fibromuscular fibbers and peritoneum leads the ovarian blood vessels. It is attached to the tubal end of the ovary and extends upwards over the iliac vessels to blend with the connective tissue and peritoneum covering the psoas major muscle.11 On axial images, the suspensory ligament is usually seen anteriorly to the psoas muscle as a triangular structure (Fig. 7). This sign seems to be the most precise in affirmation of ovarian origin, but as it may be hard to identify at first glance, it is helpful to track the ovarian vein down to the pelvis (“ovarian vein” sign) and find the suspensory ligament connecting to the ovary.12 We should remember that the right ovarian vein drains directly into the inferior cava vein, while the left one drains into the left renal vein (Fig. 8). Relying on the “ovarian vein” sign singly is considered to be less reliable as the compression of ovarian vessels by extra-ovarian masses may simulate the entrance of vessels within the mass. Of note is that the previously designated “ovarian vascular pedicle” sign, consisting of an asymmetrically enlarged gonadal vein directly joining the pelvic mass and consequently indicating that ovary as the organ of origin,13 is now considered an imprecise definition due to anatomic descriptors inconsistencies and therefore should be avoided.12
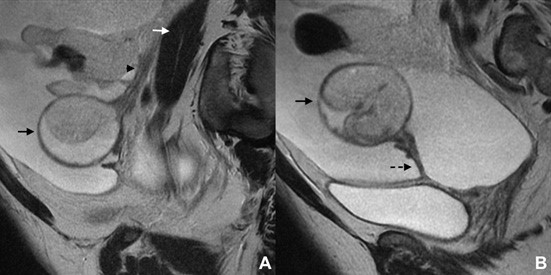
Figure 6: Cystic mature teratoma of the left ovary in a 35-year-old woman. (A, B) Sagittal T2W MR images reveal a cystic fat-containing ovarian tumour (black arrow) and severe ascites. The ovarian anchoring system may be recognized: the suspensory ligament (arrowhead) blending with the peritoneum that covers the psoas muscle (white arrow), and the utero-ovarian ligament (dashed arrow).
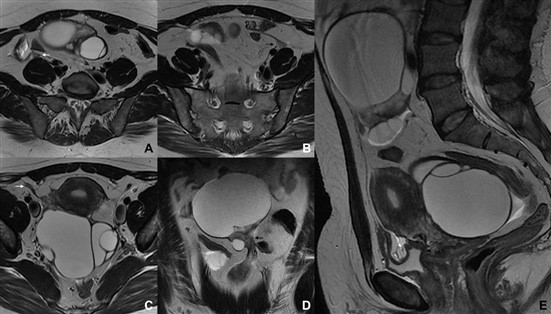
Figure 7: Bilateral ovarian serous cystadenofibroma in a 42-year-old woman. (A-C) Axial, (D) coronal and (E) sagittal T2W MR images show bilateral multilocular cystic ovarian masses. Both suspensory ligaments may be depicted as triangular structures anterior to the psoas muscles (arrows). The recognition of the suspensory ligaments allowed the identification of the origin of the tumours, the superior corresponding to the left ovary (black arrows) and the inferior corresponding to the right one (white arrows).
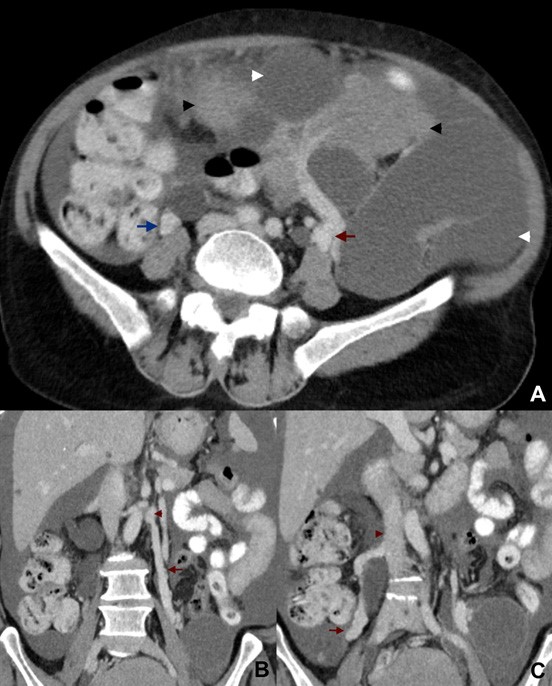
Figure 8: Undifferentiated ovarian carcinoma in a 68-year-old woman. (A) Axial and (B, C) coronal contrast-enhanced CT images show a huge left ovarian tumour with both cystic (white arrowheads) and solid (black arrowhead) portions. Both ovarian veins are easily depicted, the left one (red arrow) originating from the tumour and draining to the left renal vein (red arrowhead), and the right one (blue arrow) enlarged by reflux and draining to the inferior cava vein (blue arrowhead).
When a mass deforms the edges of ovarian parenchyma into a “beak” shape, it is likely that the mass arises from the ovary. This finding is classically known as the “beak” sign. The edges of normal parenchyma are seen extending and almost wrapping around the tumour (Fig. 9). The recognition of at least some normal ovarian tissue, what is particularly hard in large tumours, is mandatory. Otherwise, this sign will not be detectable.2
The “phantom organ” sign means that the ovary cannot be detected. It is particularly relevant in cases of large ovarian tumours that preclude the identification of ovarian parenchyma and other signs like the “beak” sign.2 In the absence of previous surgery, the inability to recognise the ovary itself may be a clue to detect ovarian origin, however this sign has a low specificity particularly in cases of huge retroperitoneal masses that may engulf the ovaries.
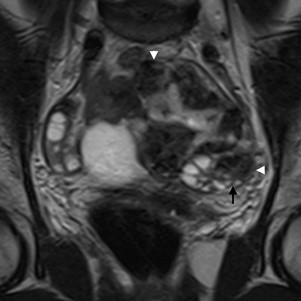
Figure 9: Fibroma of the ovary in a 34-year-old woman. Coronal T2W MR image depicts a large tumour of the left ovary, with extensive dark solid tissue (white arrowheads) and a hyperintense central area probably reflecting oedema. The normal ovarian parenchyma shows several follicles and is deformed into a “beak” shape by the tumour (black arrow).
In the particular case of peritoneal inclusion cysts, the ovaries may be found entrapped by a fluid collection (Fig. 10). The so-called “ovary within a cyst” sign highly suggests a peritoneal origin. It mostly occurs due to reactive mesothelial cells proliferation in the set of inflammatory, surgical or traumatic insult to the peritoneum.3
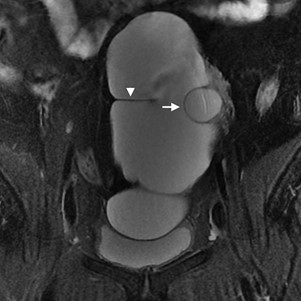
Figure 10: Peritoneal inclusion cyst in a 44-year-old woman. Coronal fat-suppressed T2W MR image shows a huge, homogenous cystic locule conforming to the peritoneum, with no well-defined wall or septa. A transverse peritoneal fold is seen mimicking a septum (arrowhead). The left ovary (with a dominant follicle) is entrapped within the inclusion cyst (arrow).
Is it from the uterus?
When a pelvic mass is clinically palpable or imaged with ultrasound, it is usually easy to determine whether the mass arises from the uterus. However, in some cases, it may be difficult to decide whether a pelvic mass close to the uterus represents an exophytic uterine leiomyoma or a mass arising from the broad ligaments or the adnexa (mainly fibromas and fibrothecomas). This mass is therefore undetermined and an MRI should be the performed. Identifying an ipsilateral ovary separated from the mass confirms a non- ovarian origin (Fig. 11). However, if one or both ovaries are hard to identify (mainly in cases of ovarian parenchyma atrophy after menopause), the radiologist must rely in other associated signs that are clues to precisely detect the uterine origin of the lesion.
The “bridging vascular sign” describes the presence of curvilinear tortuous signal void vascular structures on MRI crossing between the uterus and the mass denoting uterine origin (Figs. 12 and 13). This sign can also be extrapolated to pelvic enhanced MDCT or Doppler ultrasound.14
The “claw” sign is seen in several organs and tumours, and refers to the depiction of sharp angles on the edges of the mass. These angles are shaped by the surrounding normal parenchyma. In the case of a uterine lesion, it corresponds to projections of the myometrium surrounding a leiomyoma with a high degree of certainty (Figs. 11 and 13).15
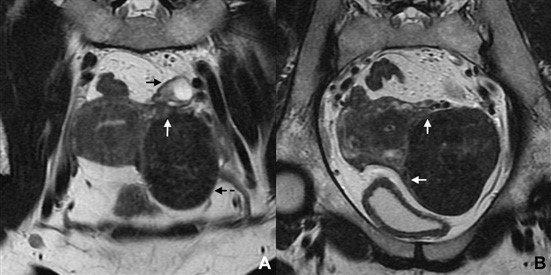
Figure 11: Subserosal leiomyoma in a 49-year-old woman. (A, B) Coronal T2W MR images depict a large subserosal leiomyoma protruding into the left adnexal area (dashed arrow). Projections of normal myometrium are seen surrounding the leiomyoma, the so-called “claw” sign (white ar- rows). The left ovary is independent and easily depicted (black arrow).
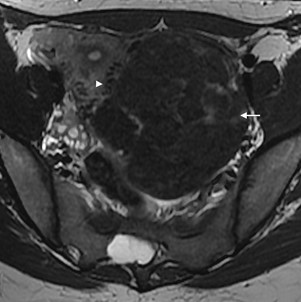
Figure 12: Subserosal leiomyoma in a 31-year-old woman. Axial T2W MR image shows a huge subserosal leiomyoma protruding into the left ad- nexal area (arrow). In the interface with the myometrium, some signal void dots may be seen (arrowhead). It corresponds to the “bridging vessels”.
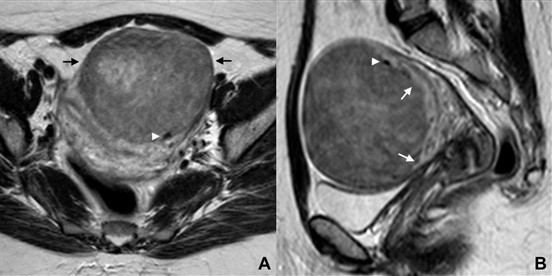
Figure 13: Subserosal leiomyoma in a 44-year-old woman. (A) Axial and (B) sagittal T2W MR images show a large, anterior subserosal leiomyoma (black arrow). Myometrial projections are seen surroun- ding it, resembling a “claw” (white arrows). The “bridging vessels” sign is also recognized (white arrowhead).
Is it from the Fallopian tube?
The Fallopian tubes are difficult to distinguish in normal imaging examinations, but may become visible in the set of ascites or intrinsic diseases. While ascites clearly outlines its contours, intrinsic tube diseases tend to distend the lumen consequently delineating walls and folds.
Hydrosalpinx, a distended fluid-filled tube, may be recognized as a cystic, tubular-shaped mass with well- defined walls. Two signs are typically found in hydrosalpinx and helps differentiating it from peritoneal, ovary or para-ovary originated cysts. The “waist” sign results from the existence of plicae and folds, and the “beak” sign corresponds to a focal narrowing of the tubular structure that produces an angular contour (Fig. 14).16-18 Note that this “beak” sign is different from the one that was described in the ovary section.
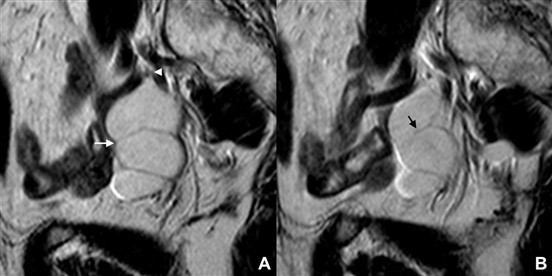
Figure 14: Chronic hydrosalpinx in a 68-year-old woman. (A, B) Sagittal T2W MR images reveal a fluid-filled, tubular-shaped structure, representing a distended Fallopian tube. Both the “waist” sign (white arrow) and the “beak” sign (white arrowhe- ad) are depicted. An incomplete fold protruding into to the lumen may also be seen (black arrow)
The “cogwheel” sign and the “beads-on-a-string” sign were initially described in ultrasound examinations, but are easily transposed to cross-sectional imaging. The former is seen when a fluid dilated tube folds upon itself typically resulting in the visualization of thick incomplete septa (Fig. 15), while the later corresponds to a dilated tube with thin wall and small round projections.19 These signs were classically described in the set of inflammatory or infectious diseases, but may be also found in neoplastic diseases (particularly the “cogwheel” sign).
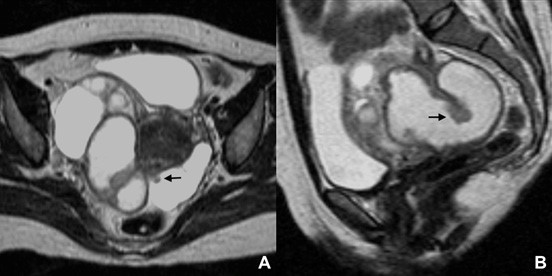
Figure 15: Tubo-ovarian abscess in a 48-year-old woman. (A) Axial and (B) sagittal T2W MR images show a right distended, fluid-filled Fallopian tube, presenting thickened incomplete septa (arrows), the so-called “cogwheel” sign.
The sausage-shaped appearance is one of the most specific signs seen in Fallopian tube originated tumours. In the set of solid enhancing component, the diagnosis of carcinoma is highly suggestive (Fig. 16).16
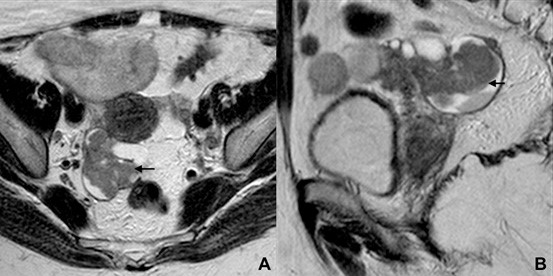
Figure 16: Fallopian tube carcinoma in a 74-ye- ar-old woman. (A) Axial and (B) sagittal T2W MR images depict a tubular, sausage-shaped structure in the right adnexal area, folding upon itself, with solid tissue within its lumen (arrows).
Finally, two exclusive MRI signs reflect the inhomogeneity of the fluid within the lumen of the tube. The “synechiae” sign refers to the presence of linear strands within the lumen of the tube probably related to fibrinous bands due to prior inflammation. On the other hand, the “amorphous shading” sign corresponds to the loss of signal intensity of the fluid from T1W to T2W images. It is typically seen in the set of purulent or haemorrhagic content (Fig. 17).16,17
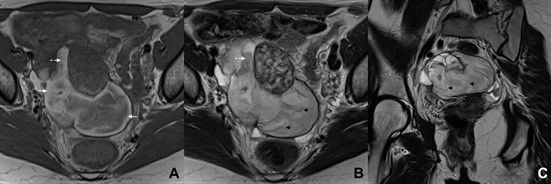
Figure 17: Tubal pregnancy in a 33-year-old woman. (A) Axial T1W MR image shows a distended tubular, fluid-filled structure in the right adnexa. Hyperintense areas may be seen at the periphery of its lumen (arrows), corresponding to blood. (B) Axial and (C) coronal T2W MR images better depict the distended tube, recognizing areas of “shading” (dashed arrow) and the “synechiae” sign (black arrowheads).
Conclusion
Female pelvic organ-confined and small-to-medium sized tumours are typically easy to depict on imaging studies. However, large female pelvic tumours may become unnerving as normal anatomy is changed by invasion, compression or displacement. Pelvic cross-sectional imaging either by CT or MRI allows a comprehensive approach of both normal anatomy and pathology, so the tumour’s place of origin is usually recognizable. The accuracy of this analysis may increase if the radiologist follows a rational approach. Normal structures should be first recognized. The tumour should then by classified as intra or extra-peritoneal. Finally, organ-specific signs should be assessed.