Introduction
Prostate cancer is the fourth most common cancer and the eighth cause of death by cancer worldwide.1,2
The diagnosis of prostate cancer is still centered on three main factors: serum prostate-specific antigen (PSA) evaluations, digital rectal examination, and transrectal ultrasound (TRUS)-guided biopsy. However, these biopsies typically sample the posterior peripheral parenchyma, thus missing central, transitional and more anterior tumors. Moreover, anterior prostate cancers cannot be easily palpable.3,4 In retrospective analysis of radical prostatectomy specimens pure anterior tumors account for 20% of all prostate cancers; however over 50% of specimens contain at least some tumor in the anterior prostate.5
Consequently, these cancers are frequently larger at diagnosis and carry a higher risk for the patient, and some studies have shown higher risk of positive surgical margins on radical prostatectomy.6,7
Tumors that may be missed in conventional biopsies include not only anterior fibromuscular band tumors, but also anterior horn of the peripheral zone tumors, the most anterior segment of transitional zones. Over 20% of the prostate cancers are missed or undersampled during (TRUS)-guided biopsy.8 This diagnostic uncertainty is a common concern in daily practice, which often results in additional biopsy sessions, as transperineal template biopsy, with varying success rates (range 10-40%).8,9
With the onset of multi-parametric Magnetic Resonance Imaging (mpMRI), the incidence in the diagnosis of anterior tumors significantly increased.10
Therefore, the main objective of this paper is to highlight the importance of mpMRI in diagnosis of anterior prostate cancers.
Normal anterior fibromuscular stroma (AFMS)
AFMS comprises a non-glandular gaping volume of stroma, containing a band of fibromuscular tissue, and being a wedge-shaped stromal barrier.
Anatomically, it is located anteriorly to the transition zone (TZ), occupying the anteromedial prostate from the apex through the base, overlying the prostatic urethra anteriorly, sheltering the urethra and the glandular tissue from the contiguous structures, and extending bilaterally near the anterior horns of the peripheral zone. AFMS founds up to nearly one-third of the prostatic tissue (Figures 1 and 2).11
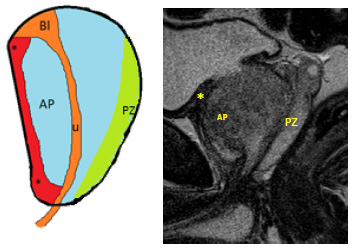
Figure 1: Normal anatomy of the prostate gland and normal appearance of the anterior fibromuscular stroma. Sagittal diagrammatic picture (A) and equivalent T2WI (B) of a normal prostate demonstrates the anterior fibromuscular stroma (*) located anteriorly, being thicker at the base and apex and thin at medium gland; peripheral zone (PZ); AP- anterior prostate.
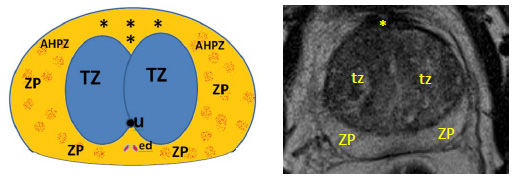
Figure 2: Axial diagrammatic representation (A) and corresponding T2-WI (B) of an axial section at the level of the medium gland, demonstrating the anterior fibromuscular stroma (*) anteriorly; the anterior horns of the peripheral zone laterally (AHPZ); the anteromedial transition zone posteriorly (tz); urethra (u); ejaculatory ducts (ed) and peripheral zone (ZP)
AFMS is variably intertwined with skeletal muscle fibers of the urogenital diaphragm at the apex and fuses with the smooth muscle fibers of the detrusor at the base of the prostate, originating its thicker appearance at the apex and the base.6,11 AFMS differs in its thickness among men, being less protuberant in individuals with increased gland size and age, probably because of the compressive effect of benign prostatic hyperplasia (Figure 3).12
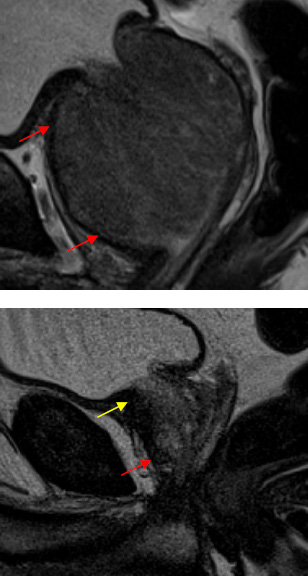
Figure 3: A - T2 sagittal WI demonstrating AFMS with reduced thickness, in this 76 year old patient with HBP (arrows). B - T2 sagittal WI showing normal thickness of AFMS, being thicker at the base (yellow arrow) and apex (red arrow).
Its relatively symmetric appearance on MRI is also in agreement with the anatomical description of normal AFMS.6,11,13
Since AFMS is formed of fibrous tissue and smooth muscle being a non-glandular gaping volume of stroma, the MR appearance (low T2 signal, low-to-intermediate DWI signal, and delayed enhancement) is similar to the MRI findings of equally composed tissues within the body elsewhere, like the bladder wall, being a result of its histologic structure.14 According to its non-glandular composition, it is rare to find a pure AFMS tumor. It may rises from adjacent parenchyma, namely from the anterior horns of peripheral zone and the contiguous transitional zones. Due to its appearance on MRI, prostate cancers can be obscure. Its distinction from prostate cancer is crucial not only to avoid unnecessary biopsies and surgeries but also to prevent missing clinically relevant anterior prostatic cancers.
The role of MRI
High-quality mpMRI became a central tool in the diagnosis, classification and treatment planning of prostate cancer.15,16,17 The recommended protocol18 includes T2- weighted anatomical images combined with functional imaging methods such as Diffusion-Weighted Imaging (DWI), Dynamic Contrast Enhanced Imaging (DCE) and T1-weighted image from renal hila to pelvic brim. Besides these sequences had demonstrated a restricted accuracy if considered individually, their association has shown a favorable performance in the assessment of prostate cancer.19
A study performed by Shinmoto et al., highlighted the importance of mpMRI in the diagnosis of anterior prostate cancers. The authors found that the sensitivity of combined T2-weighted imaging and ADC map is higher in the detection of anterior prostate cancer when compared to posterior tumors (Figure 4).20
MpMRI is usually performed in patients with either an elevated or rising serum PSA level and with one or more negative 12-core biopsies.10,21 Particularly in patients with pervious negative biopsies, it is more probable to find anterior tumors.
Detecting anterior cancers on mpMRI may also be difficult because of the concomitance of normal anatomical structures that can simulate cancer in this location. Actually,
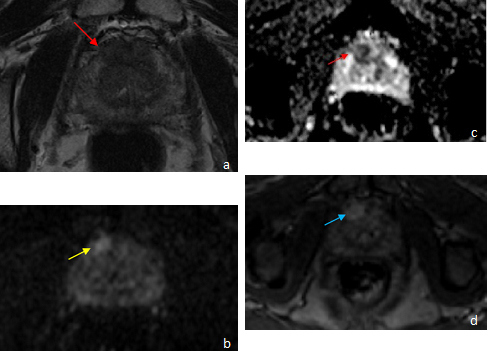
Figure 4: Axial T2-WI (a), DWI b-1500 (b), ADC map (c) and T1-VIBE after administration of gadolinium (d), revealed anterior fibromuscular band tumor, measuring 16 x 10 mm, ill-defined, hypointense on T2- WI and ADC map (red arrows);with restriction (yellow arrow) and early enhancing after contrast administration (blue arrows). This lesion was categorized as Pi-Rads 5. Histopathologic results revealed anterior prostatic adenocarcinoma.
AFMB demonstrates low signal ADC sequences, thus mimicking malignant tumors, and adenomas, localized in the contiguous transitional zones, can show restriction to diffusion.
Besides preoperative tissue diagnosis of prostate cancer has been achieved using TRUS-guided biopsy, this technique is imperfect due to its relatively low sensitivity in the diagnosis of prostate cancer.22,23 Anterior prostate cancer, is frequently missed even with typical 10- to 12-core extended systematic biopsies.24,25
MRI-targeted biopsy has been developed over the last years and has demonstrated increased in the diagnosis of clinically significant prostate cancer even performing fewer cores.26,27 Particularly in anterior prostate tumors, diagnosing rates, grade, classification and measurement of cancer per core were demonstrated to be value-added with MR-targeted biopsy in comparison with 12-core systematic biopsies.26
Watanabe et al.28,29 demonstrated an elevated positive predictive value for anterior prostate cancer using a targeted biopsy strategy based on an ADC map. Another recent study from Volkin et al.30 reported that MRI- ultrasound fusion-guided biopsy diagnoses considerably more anterior prostate cancers in contrast with TRUS- guided biopsy. With the introduction of MRI-ultrasound fusion-guided biopsy and of MRI-guided biopsy, there has been a progressive interest in describing the morphologic and imaging features of anterior prostate tumors.28,29,30
A study comparing mpMRI then targeted biopsy vs standard transrectal biopsy, found that the MRI was superior at limiting the amount of men who needed biopsies and the discovery of more clinically significant anterior prostate cancers.31 Moreover, in patients with persistently elevated PSA and negative TRUS biopsy, mpMRI then targeted biopsy has a significantly better detection in clinically significant cancers, when compared with saturation biopsy (45.5 versus 24.4%).32
Normal AFMS vs anterior prostatic cancer
AFMS carries a significant challenge in the diagnosis of anterior prostate cancers because its T2 and ADC appearance is similar to that of prostate tumors.33
One study performed by Ward et al34 demonstrated that several MP-MRI findings can be helpful in this distinction, emphasizing the enhancement pattern. Normal AFMS revealed late and progressive enhancement, in contrast to anterior cancers which frequently demonstrate early enhancement with delayed washout. This enhancement pattern can be easily acknowledged qualitatively by relating their enhancement to the normal transition zone, being also revealed on the DCE curve types. It is known that 90% of normal AFMS demonstrated a type 1 curve (characterized for progressive enhancement) which is rarely and usually not present in any of the anterior prostatic cancers. Therefore, this study suggested that the existence of a type 1 curve significantly declines the probability of cancer, even in the existence of low signal on T2WI and ADC.
In PI-RADS version 2, DCE is not comprised in the scoring of transition zone lesions and performs only a secondary role in peripheral zone lesions. However, this paper shows that DCE may be a crucial element for assessment of this subgroup of tumors in the anterior prostate (Figure 5).
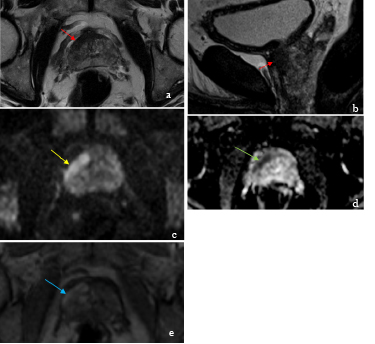
Figure 5: Axial and sagittal T2-WI (18), DWI b-1500 (c), ADC map (d) and T1-VIBE after administration of gadolinium (e), revealed a tumor localized at anterior fibromuscular band and anterior horn of right PZ (yellow arrows), with a24 x 8 mm, ill-defined, hypointense on T2-WI (red arrows) and ADC map (green arrow); with restriction (yellow arrow) and early enhancing after contrast administration (blue arrow). This lesion was categorized as Pi-Rads 5. Histopathologic results revealed anterior prostatic adenocarcinoma.
The previous study also highlights the fact that high b-value diffusion-weighted MR images also appear to be helpful in the distinction of normal AFMS from anterior cancer (Figure 6). In the great majority (90%), AFMS was hypointense to the normal peripheral zone, in contrast with anterior prostate cancers, which were hyperintense, which means they are restrictive (81%).34 Moreover, the previous study also concluded that besides the overlying values, both mean and tenth percentile quantitative ADC values were also significantly different among AFMS and cancer. These differences may be useful in consistently discriminating AFMS from anterior cancers.
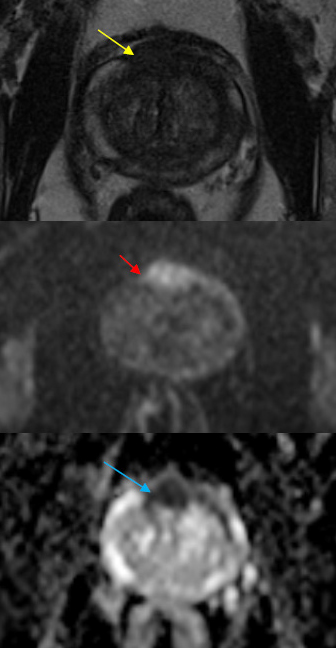
Figure 6: Axial T2-WI (a); DWI b-1000 (b) and ADC map (c), demonstrating hypersinal on T2WI (yellow arrow) and DWI (red arrow) and hypossinal at ADC map (blue arrows), measuring 25 mm of great diameter. This lesion was classified as Pi-Rads 5. Pathological results revealed anterior prostatic adenocarcinoma.
In addition, another recent study35 from Panebianco et al., demonstrates that anterior fibromuscular stroma does not frequently demonstrates enhancement on DCE nor significant restriction on DWI, even though low ADC values can be observed occasionally, because of pre- existing low T2-signal areas rather than a true restriction. In contrast, a study performed by Jinxing et al,36 suggested that key factors for detecting normal AFMS are: significant T2 hypointensity, inferior than that characteristically expected for prostate cancer; absence of early enhancement with washout, its symmetrical and midline or near midline location and its anatomically continuity with the remain anterior fibromuscular stroma.
There is variability among studies regarding imaging key clues to distinguish AFMS from anterior prostate cancer. Main differentiating factors of principal studies are described in Table 1.
Therefore, it is recommended35 to access all the available sequences and planes, including coronal and sagittal planes. A supplementary acquisition plan is of utmost importance, as it can confirm the symmetry and continuity of the hypertrophic anterior fibromuscular stroma with the benign tissue. The T2WI morphological evaluation, combined with absence of positive findings at DWI and DCE, is able to exclude the existence of clinically significant prostate cancer.
Table 1: Main points of three different studies in differentiating normal AFMS from anterior prostatic cancer
| Study | AFMS | Anterior prostatic cancer | |
|---|---|---|---|
| Ward et al., 2016 | Contrast enhancement pattern | Late and progressive | Early with washout (more distinguishable factor) |
| DWI | Hypointense ( in contrast with PZ) | Hyperintense (in contrast with PZ) | |
| ADC | Higher | Lower | |
| Panebianco et al., 2018 | Contrast enhancement pattern | Rarely enhances | - |
| DWI | Without significant restriction | - | |
| ADC | Low ADC values can be observed | Hypointense | |
| Jinxing et al., 2017 | T2-WI | Significant hypointensity | Hypointensity, superior than that observed in AFMS |
| Contrast enhancement pattern | Absence of early enhancement with washout | Early enhancement with washout | |
| DWI | - | - | |
| ADC | Low ADC values | - | |
Conclusion
The diagnosis of prostate cancer located at the posterior gland is commonly straightforward through the use of high PSA levels and TRUS-guided biopsy results. In contrast, anterior prostate cancer is frequently difficult to diagnose on TRUS-guided biopsy typically because of its location.24,25
Therefore, anterior prostate cancer is often diagnosed only after recurrent negative biopsies. Accordingly, MRI gets a significant role in more precise lesion localization, particularly in the diagnosis of anterior prostate cancer.
Prebiopsy MRI should be performed to avoid missing clinically significant cancer in the anterior gland, a problem inherent in the use of TRUS-guided biopsy.37














