Introduction
Fade Mansion, 36 Thomas Street, Dublin, was built c. 1728 (Hickey, 2018), in the early classical style, as a home to banker Joseph Fade (Ireland, 2013). In 2018, restoration works were carried out to turn the Mansion into student housing. This paper is based on a report commissioned by National Monuments, to investigate the original materials in order to assist with decisions regarding the conservation of the structure. Fade Mansion can be considered an early brick occurrence. Bricks in Ireland appeared relatively late when compared to other European countries. The earliest reference to brick is found in the murage taxes of medieval towns such as Kilkenny (1283), Drogheda (1296) and Youghal (1358), and early brick structures are sporadically found, but the use of brick was limited until the mid-17th century (Pavía and Bolton, 2000). In Dublin, the first brick-maker is recorded in 1599 and brick use is noted in houses and chimneys of similar date (Roundtree, 2007). Bricks became increasingly popular during the Georgian period (1714-1830). The first regulations were introduced in 1729 and, by the mid-1800s, brick making was widespread (Roundtree, 1999). The Fade Mansion bricks are investigated with established analytical techniques (petrographic microscopy, X-ray diffraction (XRD) and scanning electron microscopy (SEM)) that have been used to gather evidence of ceramic technologies and raw material sources for decades.
Firing, applied to a particular clay, results in a distinct state of sintering which is characterised by a specific mineral association and a particular extent of vitrification, that depend on the firing temperature and duration, and the composition of the raw clay. A high firing temperature maintained for a short time results in a similar state of sintering than a lower temperature held for longer (Heimann, 1982; Dunham, 1992). Fluxes such as carbonates, alkalis and iron phases, such as hematite, can enhance phase reactions during firing, so that transformation can occur at lower temperature (Heimann, 1982; Diminuco et al., 1996).
The mineral associations in the bricks were determined with XRD. Firing temperatures were deduced based on work by former authors. Clay minerals transform into other silicates at approximately 900°C (Dunham, 1992), but illite can remain up to 950°C in a non-calcareous clay (Maggetti, 1982). Mica breaks down at 950°C, decomposing into iron oxide and glass at 1100°C (Cole and Segnit, 1963). Plagioclase and potassium feldspar can remain up to 950 and 1050°C, respectively; and feldspar transforms into mullite at over 1050°C (Maggetti, 1982). Spinel and hematite form between 900 and 1150°C (Maggetti, 1982; Veniale, 1994); spinel appears at 1000-1100°C (Brindley and Maroney, 1960). Quartz can remain untransformed at temperatures up to 1100°C (Maggetti, 1982); at 1200°C, it rarely shows incipient change to cristobalite, but at 1250°C, reabsorption at the margins and partial conversion to cristobalite are evident (Cole and Segnit, 1963).
The extent of vitrification was determined semi-quantitatively with a SEM-EDX system focusing on the quantity of glass and the pore structure, which depend on the firing temperature and duration (Kingery, 1982; Tite and Maniatis, 1975; Freestone and Middleton, 1991). Tite and Maniatis (1975) define four groups corresponding with the SEM morphology. In the NV group, there is No Vitrification, and the material is similar to the raw clay; the 2nd group exhibits Initial Vitrification (IV), with a network of discontinuous glass filaments; in the 3rd group (III), the glass begins to form larger areas, while the 4th group exhibits Continuos Vitrified (CV) surfaces with isolated pores replacing the original structure. The IV structure develops in the range 800-850°C (for non-calcareous clays in oxidising atmospheres (Maniatis and Tite, 1981), and an increase of 150°C separates the IV from the continuous vitrification stage (Maniatis and Tite, 1981; Tite et al., 1982). Experiments by Cole and Segnit (1963) place the IV threshold at higher temperature: a small amount of glass is present at 1050°C and bloating occurs at 1250°C indicating extensive melting.
The firing temperature, determined by texture SEM analyses, is dependent on the amount of flux in the clay. According to Tite and Maniatis (1975), the same extent of vitrification can be achieved by firing a calcareous clay at low temperature (850°C) or a refractory, non-calcareous clay at higher temperature (950-1000°C).
With respect to the firing method, a significant amount of bricks in Ireland were fired in non-permanent structures known as clamps (Boate, 1652). Clamps (or open fires) are characterized by fast heating rates and short times at top temperatures. According to Tite (1995), maximum temperatures fluctuate from 500-900°C, with a high proportion in the 600-800°C range, and variations of several hundred degrees occur in a single firing. In contrast, temperature variation is limited in kiln firing, and top firing temperatures are usually higher, with considerable ware reaching 750-950°C.
This paper also attempts to determine the type and origin of the raw material used to make the bricks. A variety of raw materials have been used to produce historic bricks in Ireland, including glacial, cannal, alluvial and lake sediments, Carboniferous shales and Triassic marls. Glacial tills (or boulder clays) were a common historic source of raw material for brick making in the east, the south-east, and counties Mayo and Leitrim (Pavía and Bolton, 2000), and the occurrence of abundant sand and occasional rock clasts are typical features of Irish bricks made with boulder clay (Pavía, 2006). Petrographic analysis was used to identify local or foreign production (Freestone, 1995). The type of the raw material were resolved, including the primary or pre-firing minerals; the minerals formed during the firing process and the post-firing or secondary minerals formed during burial or outdoor exposure (Maggetti, 1994; Maggetti, 1995).
Materials and methods
Representative samples of bricks were collected from Fade Mansion’s façade (Figure 1), and from an earlier structure at the back (Figure 2). The structure was probably built before 1634 as the window shapes and positions match a structure that appears in a 1634 drawing (representation of Thomas Court included in Clarke (1990), Paul Duffy of Irish Archaeological Consultancy, personal communication, 2018). The sampling intends to represent the variable nature of the bricks and their current condition. It was based on the brick’s colour, hardness and condition, and the presence of black core, cinders and lamination. The back wall bricks are bigger, but the colours are similar to those in the façade (red to orange, occasional yellow). The back wall bricks hardly include any pebbles or lamination. However, this could not be confirmed as access was limited.

Figure 1 Fade Mansion during restoration work in 2018, showing the original brick fabric and the sampling. Published with the permission of Neil Crimmins of Cathal Crimmins Architects.
The fabric of the back wall and the façade differ significantly. In the back wall, the window heads are rough arches with voussoirs of uncut brick resulting in wide joints whereas, in the façade, the voussoirs of the flat arches at the window heads are rubbing bricks (Figure 3) which were accurately cut to produce fine joints (2-5 mm). The architectural bond is also different. In the façade, alternate headers and stretchers were laid in each course (Flemish bond) (Figure 4) while, in the back wall, the bond is inconsistent: two header courses per stretcher course changes into two stretchers per header course (Figure 5), but several headers followed by several stretchers also appear in a single course.

Figure 3 Comparison of the fabric of the window heads in the back wall (left) and the façade (right). Photographs by Sara Pavía.
The study was based on the application of several analytical techniques of the physical sciences as explained below. The study followed the methodology established by previous authors (Maggetti, 1982; Veniale, 1994; Kingery, 1982; Tite and Maniatis, 1975). The mineralogical composition was analysed with X-ray diffraction (XRD) using the powder method, with a Phillips PW1050/80 goniometer and a PW3313/20 Cu k-alpha anode tube, at 40kV and 20mA. All measurements were taken from 3 to 70 degrees (2θ) at a step size of 0.02 degrees/second. In powder diffraction, a randomly oriented, fine powder is required for phase identification. Therefore, bricks fragments were crushed and ground into a fine powder. The mineral content was approximately quantified with the relative intensity of the peaks in the XRD scan (limit of detection c. 5%). The microstructure and elemental chemical composition were obtained with a scanning electron microscope (SEM) coupled with an energy dispersive X-ray spectrometer (EDX). The bricks’ fragments were carbon-coated to perform the analyses. Micrographs (secondary electrons) and spectra were captured at 20KV. Brick specimens were also selected for thin-section preparation and petrographic analysis with transmitted polarised light, using objectives of 2X, 10X, 20X and 40X magnifications (Table 1).
Firing temperature and atmosphere based on the xrd mineral assemblage
The XRD results (Table 2) evidenced that the bricks were made with a non-calcareous earth. Based on their mineral associations, the bricks are graded from highest to lowest firing temperature. The highest firing temperatures are inferred from the mineral assemblages in the black cores and cinders (B1, B6, B8), which include quartz polymorphs cristobalite and tridymite (>1000°C), cordierite, spinel and magnetite, indicating that temperatures reached over 1250°C. The assemblages in the black cores (XRD1) indicate that they probably reached the highest temperatures at which hematite and magnetite transformed into spinel (FeAl2O4 - probably hercynite), which forms at 900-1100°C (Maggetti, 1982; Cole and Segnit, 1963; Brindley and Maroney, 1960); and most feldspar disappeared (1050-1100°C). It seems that the decomposition of feldspars has formed cordierite (2MgO.2Al2O3.5SiO2) and spinel (FeAl2O4) rather than mullite (appears over 1050-1200°C).
Table 2 Mineral assemblages determined with XRD. T°- firing temperature. XX-Abundant (~40%); X-subsidiary (40-15%); (X)-minor (15-7%). Q-quartz (SiO2); H-hematite (Fe2O3); Mg-magnetite (Fe3O4); Sp-spinel (FeAl2O4); T/Cr-trydimite/cristobalite (SiO2); C-cordierite (2MgO.2Al2O3.5SiO2); Fd-feldspar (Na/Ca/K); M-mica; I-illite/clay; Z-zeolites; Il-ilmenite (FeTiO3); G-gypsum (CaSO4.2 H2O); Ca-calcite (CaCO3).
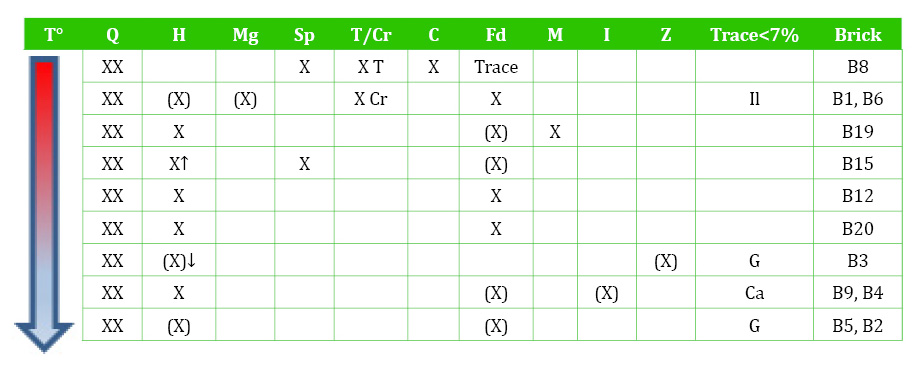
Bricks B12, B15, B19, B20 reached over 950°C as indicated from the absence of clay minerals and the sintering of hematite and spinel (Maggetti, 1982; Brindley and Maroney, 1960). The lowest temperature bricks (B2, B3, B4, B5, B9) (<950°C) include clay minerals, zeolites and sulphate (gypsum). The clay minerals (mainly illite) are likely the remains of sedimentary clay that has not transformed during firing. However, the zeolites and gypsum might be secondary, resulting from weathering on outdoor exposure. Therefore, they were further studied with petrography and SEM.
The constant presence of hematite agrees with the predominant red/orange brick colour and indicates an oxidising atmosphere, which was consistent throughout the firing. In an oxidising atmosphere, a non-calcareous clay is likely to produce red colour as the iron remains as hematite rather than forming calcium and iron silicates (Firman and Firman, 1967; Firman, 1994). From the XRD assemblages (Table 2), it can be deduced that some hematite was naturally occurring in the raw material, and that the amount increases with the firing temperature up to a certain temperature, after which it transforms into spinel (B8, B15).
Petrography and structure of the low-temperature brick
According to the mineral associations determined with XRD, bricks B2, B3, B4, B5, B9 reached the lowest firing temperatures. Their common characteristics on hand sample are a colour varying from orange to red and yellow, occasional material loss, softness, lack of cohesion and sedimentary-like lamination (Figure 6).

Figure 6 Typical appearance of the low temperature specimens. Left: rubbing bricks form the façade (B3). Right: B9 from the back wall. Photographs by Sara Pavía.
The petrographic microscope shows abundant pre-firing minerals and rocks with little or no transformation, including chert, silt-sized quartz, K-feldspar, and minor amounts of other detrital minerals (Figure 7). However, pre-firing minerals sporadically show transformation, and this is coupled to an incipient sintering in the matrix (Figure 8). The matrix varies from opaque to birefringent (Figure 7), and contains abundant iron oxides, scattered fine micas or illite flakes, and occasional isotropic glass (Figure 8).
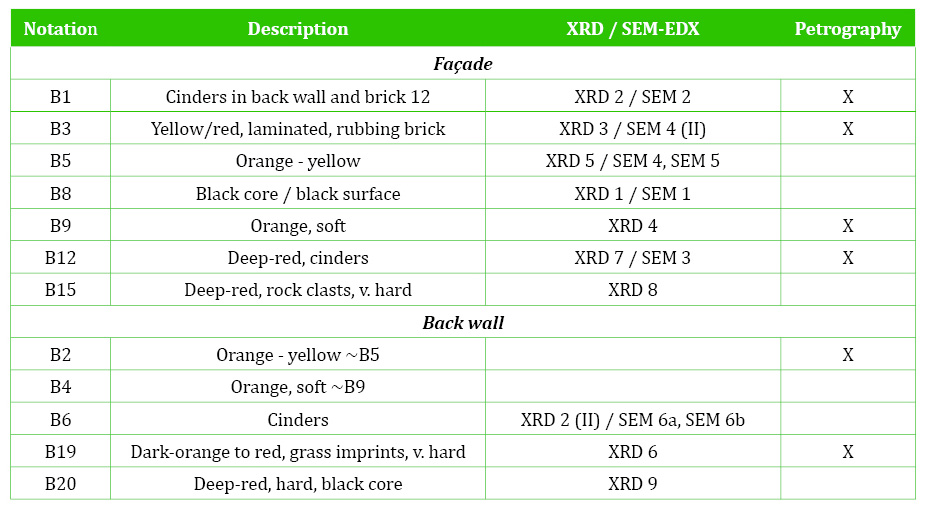
The SEM shows an open structure with occasional sintering and transformation, closer in appearance to the porous structures of sedimentary rocks than to ceramic materials (Figures 7 and 9). The SEM structure corresponds to the initial vitrification (IV) stage of Tite and Maniatis (1975), which develops at temperatures in the range 800-850°C; 1050°C according to Cole and Segnit (1963) and at 800-1000°C according to Veniale (1994). The SEM showed recrystallized calcite (CaCO3) and gypsum (CaSO4.2H2O) (Figures 10 and 11), evidencing that some of the phases determined with XRD are secondary, formed on outdoor exposure.
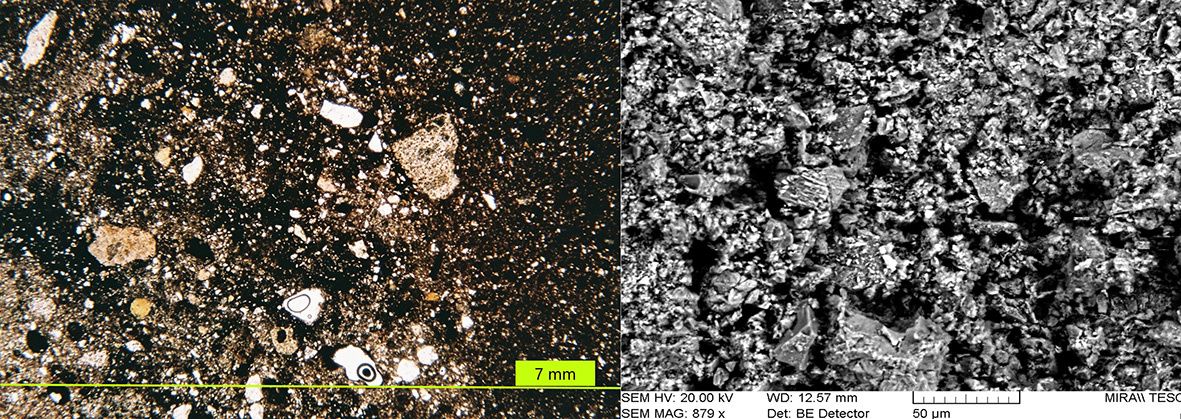
Figure 7 Typical structure of the low-temperature specimens (B9). Left: Petrographic image showing chert, and silt-sized quartz and feldspar in a matrix with abundant iron oxides and occasional opaque glass-crossed polars light. Right: SEM image showing intermediate vitrification, with an open porous structure including some glass and detrital minerals largely unchanged. Photographs by Sara Pavía.
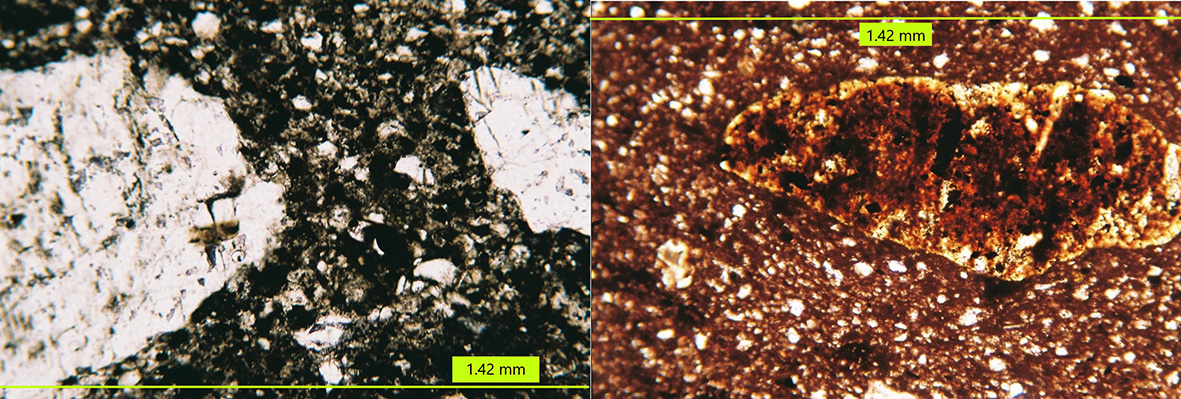
Figure 8 Left: Pre-firing minerals with some transformation and incipient sintering in the matrix (B3). Right: Coarse chert relict partially transformed by firing in B9, showing a reaction rim, depleted in iron oxides. Both images: 10X plane polarized light. Photographs by Sara Pavía.
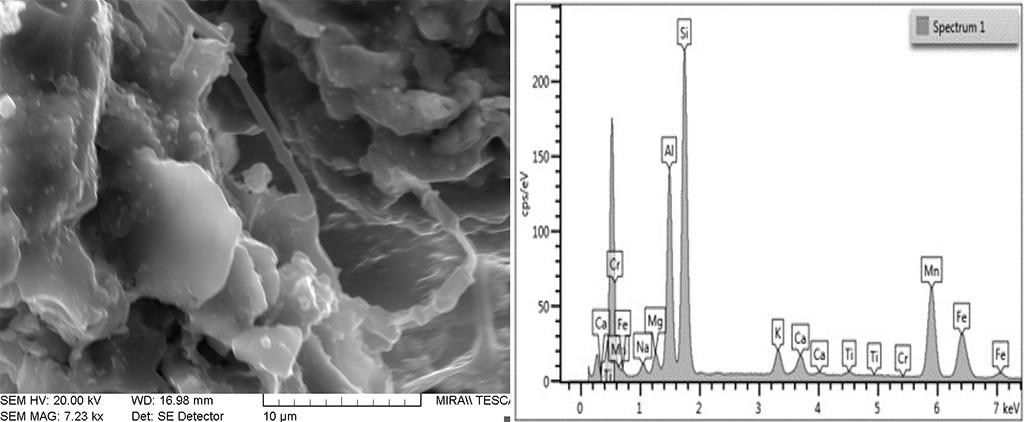
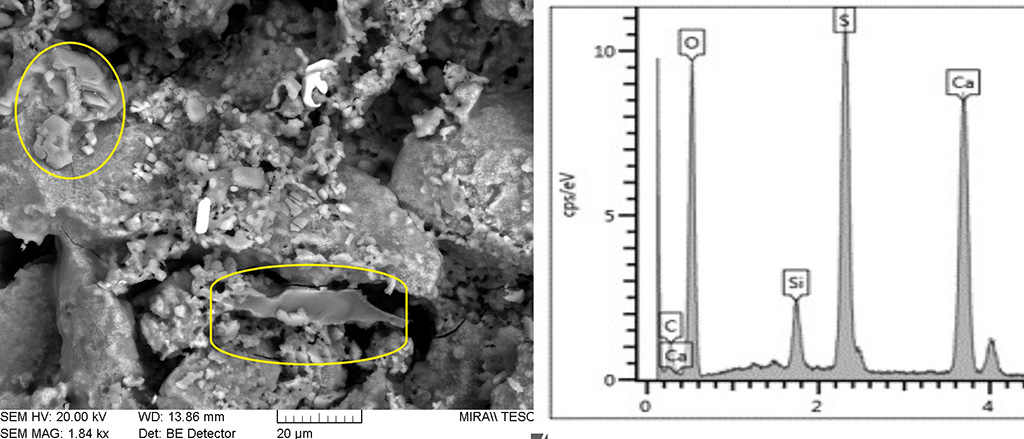
Figure 9 SEM image of an area with partial melt in a low temperature brick. The glass shows a composition similar to that in the higher temperature bricks where Si, Al, Mn and Fe predominate. Photograph and graphic by Sara Pavía.
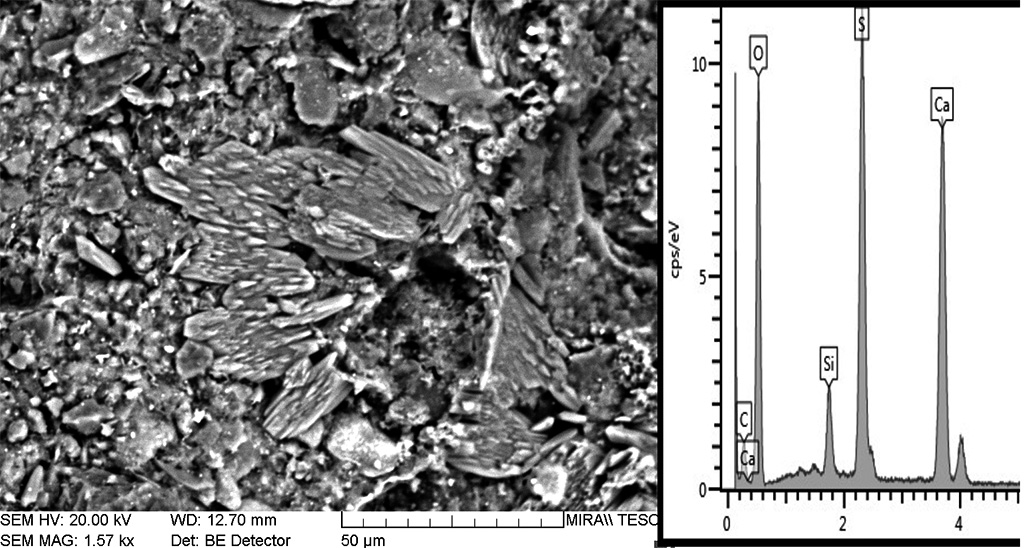
Figure 10 Secondary calcite and gypsum replacing the structure of rubbing brick B3 under the SEM. Photograph and graphic by Sara Pavía.
Petrography and structure of the high-temperature brick
According to the mineral associations determined with XRD, bricks B12, B15, B19, B20 reached high firing temperatures. On hand sample, they display a dark-orange to red colour. They are hard and cohesive and lack lamination. Some include cinders, and show mineral and rock clasts of chert, quartz, granite (>4 mm) partially transformed by firing (Figure 12).

Figure 12 Specimen B12, represents the typical appearance of the high-temperature bricks. Photograph by Sara Pavía.
The petrographic microscope showed a ceramic structure with abundant glass and crystalline, newformed pha-ses, rock clasts partially transformed by firing, sintered iron oxides (Figure 13), and mineral transformation affecting micas, quartz and feldspar (Figure 14). The micas have lost their optical properties, they are slightly birefringent and appear dark, exfoliated and partially decomposed into opaque mixtures of iron oxide and glass, and occasionally crystalline phases (possibly mullite) (Figure 14), indicating temperature over 1050°C. The SEM texture agrees with the continuous vitrification stage of Tite and Maniatis (1975), which develops at firing temperatures in the range 950-1000°C; it shows abundant glass and transition between areas with discontinuous glass and others where coalescent glass has formed continuous layers (Figure 15).
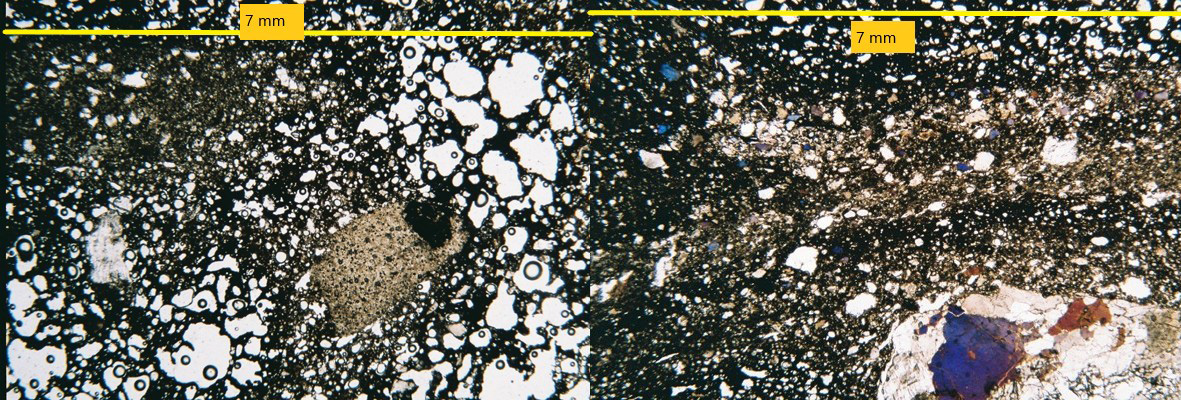
Figure 13 General structure of high temperature B12 under the petrographic microscope. Left: coarse chert relict partially transformed by firing with sintered iron oxides, surrounded by opaque glass, abundant silica segregation and bloating pores. Right: abundant opaque glass alternates with crystalline sintering. Coarse granite fractured and partially transformed (likely feldspar into cordierite). Photographs by Sara Pavía.
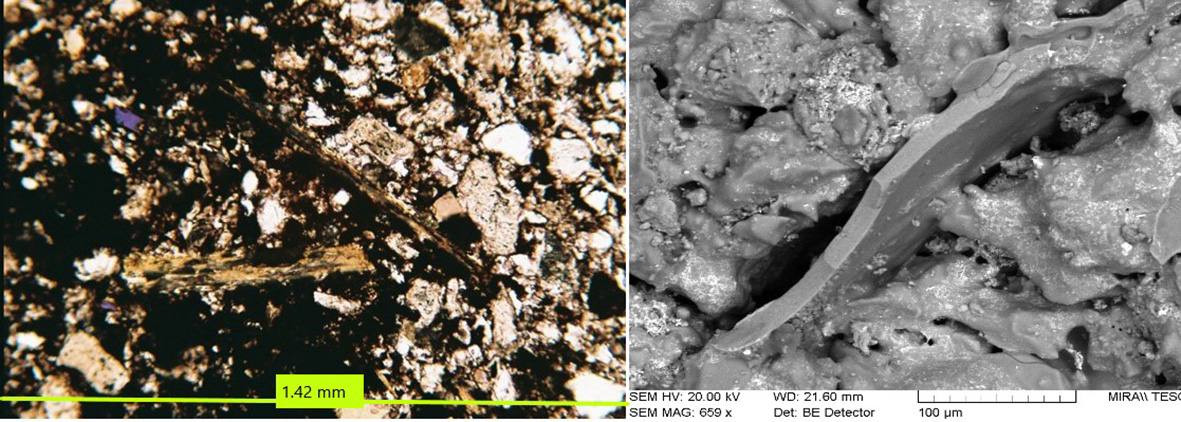
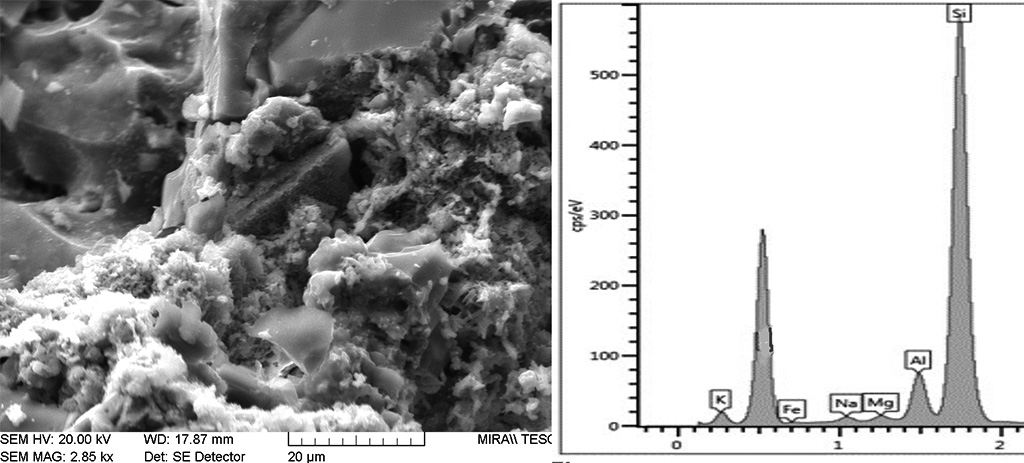
Figure 14 Delamination and transformation of phyllosilicates due to dihydroxylation at high temperatures. Left: mica exfoliated and partially transformed into iron oxides, glass and a new-formed crystalline phase, possibly mullite-polarised light. Right: SEM showing the mica cemented with glass. Photographs by Sara Pavía.
Petrography and structure of the highest temperature matrices with black core and cinders
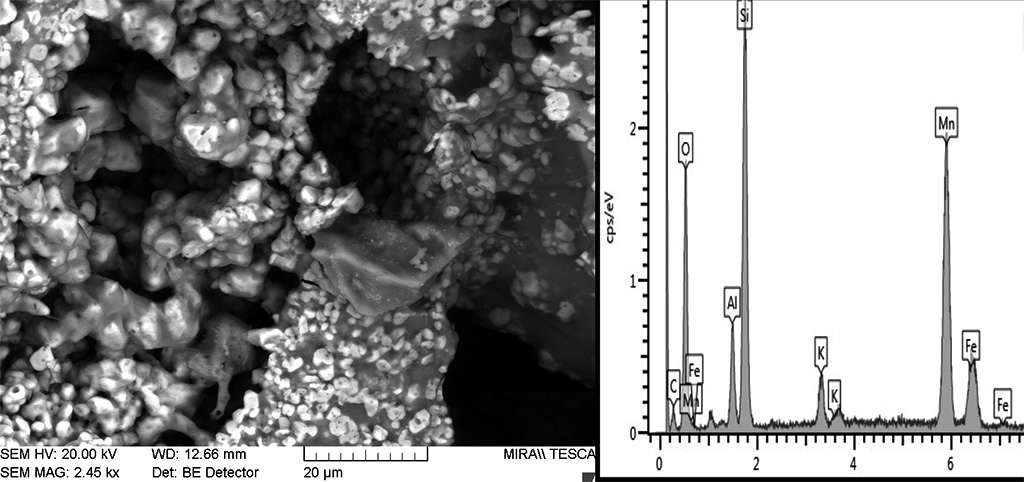
The typical high-temperature matrices with cinders and black core evidence strong sintering, including newformed crystalline phases and newformed glass and oxides minerals (Figure 16). Bloating has sporadically occurred, indicating extensive melting (Figure 17). Microcrystals of sintered manganese and iron oxides are often found associated with glass, both in the matrix and inside cinders (Figure 18) and coalescent glass can form continuous layers (Figure 16). These features indicate high temperatures c. 1250°C. The SEM structure evidenced a continuous vitrification stage with bloating which occurs when gases escape from a viscous glass at 1250°C and indicate extensive melting (Cole and Segnit, 1963; Tite and Maniatis, 1975). According to Veniale (1994) bloating usually appears at 1200-1300°C.
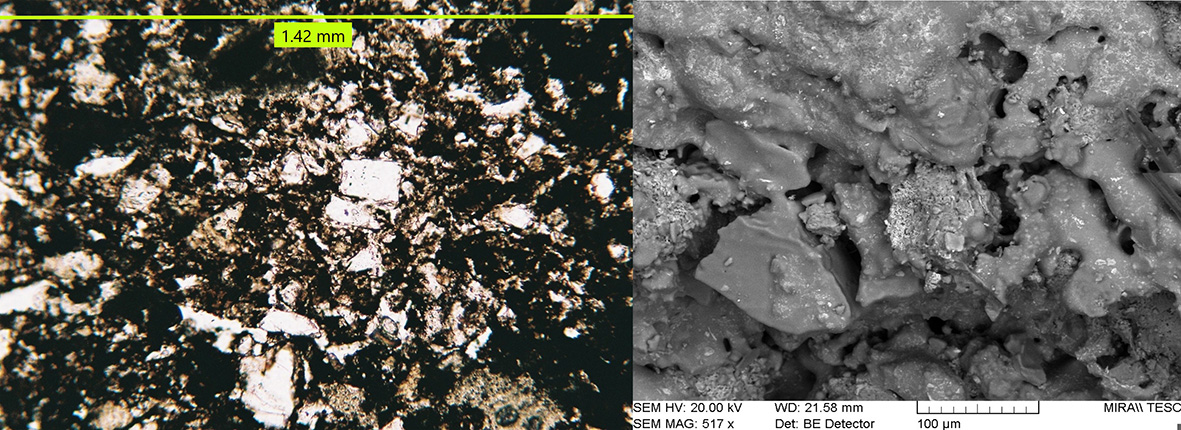
Figure 16 Typical structure of a high-temperature brick (B6). Left: Matrix with abundant new-formed crystalline phases, opaque glass and iron oxidesplane polarized light. Right: SEM image of the matrix at a higher magnification showing coalescent glass in continuous layers. Photographs by Sara Pavía.
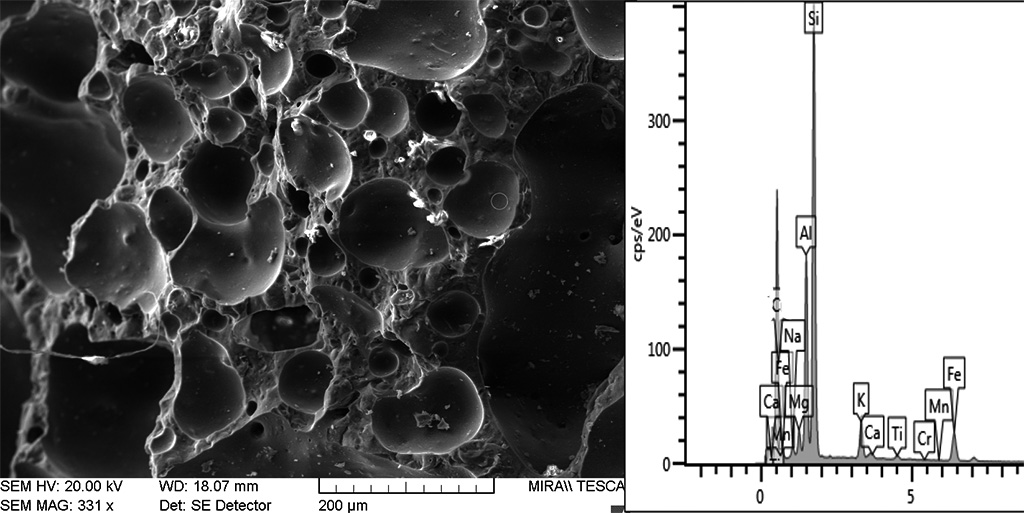
Figure 17 Continuos vitrification with bloated pores in black cinders (B12). The EDXA analysis of the bloated silica glass implies the presence of iron spinel - FeAl2O4 indicating temperatures up to 1300°C. Photograph and graphic by Sara Pavía.
Discussion
Even though their size and building fabric are different, and they were likely built 100 years apart, the results indicate that the façade and back wall bricks are similar in composition and were fabricated with a comparable raw material.
Brick provenance: type and origin of the raw material
The petrographic analysis shows a high silicate content, and the presence of rock clasts, and abundant sand and silt-sized particles. Firman (1994) states that a matrix with coarse sand and occasional pebbles is characteristic of bricks made with boulder clays or silty terrace gravels derived from boulder clays. These petrographic features, together with the lack of carbonates determined with XRD, indicate that the material used for brickmaking was a non-calcareous clay of glacial origin (also known as glacial till or boulder clay). Boulder clays are usual in Dublin, these materials were a common historic source of clay for brickmaking (Pavía and Bolton, 2000).
The bricks that reached lower temperature (e.g. the rubbing brick at the windows) include sporadic lamination, which according to Firman (1994) suggests some alluvium influence.
Fade Mansion is located on a terrace of the River Liffey, in Dublin city, a position that would agree with the use of a local decalcified boulder clay with some alluvium influence.
The rock clasts (predominantly chert and granite) are consistent with the geology of the Dublin area, hence supporting a local origin. The petrography of the brick, with abundant quartz sand and occasional rock fragments, agrees with contemporary bricks, made with local clay in Dublin and nearby areas, such as Arch Hall, built 1730, and Rathfarham Castle, built 1618-1771 (Pavía, 2006; Pavía and Roundtree, 2005).
Firing temperature
Diagnostic minerals are used to determine firing temperatures. However, the Fade Mansion bricks were made with a non-calcareous clay. In the Fade Mansion bricks, the development of glass predominates over the sintering of crystalline phases agreeing with previous authors. The only high-temperature crystalline phase detected by Maniatis and Tite (1981), in fired non-calcareous clays, was a small amount of spinel. Similarly, Tite et al. (1982) only find spinel and mullite in non-calcareous ceramics. In the Fade Mansion bricks, a firing temperature range was determined based on the presence of glass and the SEM microstructure, the mineral associations determined with XRD and the mineral transformation and newformation recorded with petrographic analyses (Table 3). The disappearance of clay minerals, the transformation of quartz, micas and feldspar, and the newformation of hematite, high-temperature quartz polymorphs, cordierite and spinels were used to establish firing temperatures.
The XRD mineral assemblages (Table 2) show that increasing temperature causes the disappearance of clay minerals (indicating minimum temperatures of 850°C) and the transformation of muscovite (starting at 900°C). The results also show that hematite is partly inherited from the original sediment and partly formed during firing. In historic Irish bricks made with non-calcareous clays, an increase of newformed hematite is typically linked to an increase in firing temperature (Pavía, 2006).
The high-temperature assemblages include: quartz polymorphs cristobalite and tridymite (>1000°C), cordierite, hematite, spinel and magnetite, indicating that temperatures reached c. 1000-1200°C. As seen in the introduction, previous authors have proven that, in non-carbonate sediments, hematite forms above 1000°C, while spinel appears at 1000-1100°C and magnetite in the range 1000-1250°C.
Quartz appears occasionally transformed in the highest temperature minerals. Quartz usually remains untransformed up to 1200°C, but at 1250°C, reabsorption at the margins and partial conversion to cristobalite can occur. The evolution of the feldspar with increasing temperature is not clear. Plagioclase and potassium feldspar can remain up to 950 and 1050°C, respectively, and feldspar transforms into mullite at over 1050°C (Maggetti, 1982). However, the only clear trend in the Fade Mansion bricks is that, at the highest temperature, feldspar has mostly disappeared (1050-1100°C).
Brick B8 reached the highest temperatures at which hematite and magnetite transformed into spinel (FeAl2O4 - probably hercynite) and most feldspar has disappeared, likely forming cordierite (2MgO.2Al2O3.5SiO2) and spinel (FeAl2O4). The high-temperature assemblages are comparable to the typical 1200°C assemblage found by Kühnel et al. (1993) in argillaceous rocks containing cristobalite, cordierite, hercynite (FeA12O4 - spinel), magnetite, anorthite, residual quartz and abundant glass.
In some specimens, the SEM revealed initial vitrification (IV) structures which, according to Maniatis and Tite (1981; 1978) develop at 800-850°C in non-calcareous clays lacking carbonate fluxes in oxidising atmospheres (at 1050°C (Cole and Segnit, 1963)). In other bricks, the SEM showed a continuous vitrification stage which suggests and increase of 150°C from the IV stage (Tite et al., 1982). Finally, the ‘hot spots’ with bloating pores in the highest temperature specimens indicate extensive melting suggesting c. 1250°C (Cole and Segnit, 1963).
The SEM structure and XRD mineral associations indicate that the rubbing bricks comprising the window heads are amongst those that reached the lowest temperature (Figure 3). Therefore, their current poor condition and serious damage are attributed to a low vitrification, which agrees with the occurrence of sedimentary features such as lamination (Figure 6), probably inherited from an alluvial influence in the original sediment (Firman, 1994). The secondary minerals replacing the ceramic structure evidenced with SEM (Figures 10 and 11) also agree with the lack of vitrification resulting in a less durable ceramic body.
Firing method
The XRD mineral associations, SEM structures and petrographic fabric indicate a wide firing temperature range (c. <800-1200°C) which agrees with the inconsistent physical properties (the bricks vary in hardness and cohesion) and is a common feature to the historic brick range.
The predominantly red and orange colours are due to the presence of ferric oxide: hematite (Firman, 1994). The constant presence of hematite in the Fade Mansion bricks agrees with the predominant red/orange brick colour and indicates firing in an oxidising atmosphere which was consistent throughout the firing.
The consistently oxidising atmosphere coupled to the high firing temperatures and the rare occurrence of black core, scum and reduction marks, suggest some control over the firing operation and hence the use of kilns. The wide temperature variation also agrees with historic kiln firing, as experimental findings have indicated that the temperatures reached in different parts of a kiln can vary as much as 100°C or more (Tite et al., 1982; Tite, 1995).
Conclusion
The analytical results evidenced that the Fade Mansion bricks were made with a silica-based, non-calcareous clay of glacio-fluvial origin, gathered locally, with a high percentage of non-plastic material. This agrees with the position of the Fade Mansion in a terrace of the River Liffey in Dublin city, and with the results of previous research on historic bricks made with boulder clay from the region. The façade and back wall bricks are similar in composition and were fabricated with a comparable raw material.
The bricks were fired in kilns in an oxidising atmosphere which was consistent throughout the firing. A firing temperature range is proposed based on the presence of glass and the SEM microstructure, the mineral associations determined with XRD and the mineral transformation and newformation recorded with petrographic analyses. The disappearance of clay minerals, the transformation of quartz, micas and feldspar, and the newformation of hematite, high-temperature quartz polymorphs, cordierite and spinels were used to establish a firing temperature range. The presence of initial vitrification structures convene on firing temperatures of c. 800°C, while the continuous vitrification structures appear in bricks were the clay minerals have disappeared, hematite has sintered and/or micas are transformed and high-temperature phases have appeared (>950-1200°C). This wide firing temperature range (c. <800-1200°C) agrees with the inconsistent physical properties (the bricks vary in hardness and cohesion) and is a common feature of historic brick. The rubbers at the window heads were probably fired at lower temperatures (so that they could be cut to build the window arches with fine joints) which resulted in low vitrification leading to weathering and material loss.