INTRODUCTION
Gestational Diabetes Mellitus (GDM) is defined as the development of chronic hyperglycemia in pregnant women without previously diagnosed diabetes, and it is reported to be one of the most frequent medical complications during pregnancy (1). In recent years, later childbearing, changes in lifestyle, unhealthy diets, and physical inactivity have contributed to the increased incidence rate of GDM, affecting 13.4% of pregnant women in 2017 (1, 2). In Portugal, the most recent data from 2016, reports a prevalence rate of 7.5%, resulting in a rise of 4.1% in comparison to 2006, and mainly in women over the age of 40 years old (3, 4). GDM is also associated with a significantly greater maternal and fetal risk related to the degree of hyperglycemia. In general, risks of GDM can include macrosomia, complications during childbirth and increased risk of developing type 2 Diabetes Mellitus later in life (1). Thus, early screening and diagnosis are crucial to assess the most effective therapy, either based on lifestyle changes or pharmacological treatment, to ultimately avoid adverse outcomes (5).
The diagnosis is established with an Oral Glucose Tolerance Test (OGTT), the gold standard method to detect glucose metabolism disorders (6). Normally it is performed between the 24th and the 28th week of gestation and can be applied in one or two steps since there is still no international agreement on which of the methods is more advantageous (7). However, this method presents various side effects, thus some studies have tested alternative approaches, one of them being the use of a mixed meal tolerance test (MMTT), which can be a less expensive method and better accepted by pregnant women (8-10).
Currently, data on the association between the 2-hour glucose value after oral glucose ingestion and after standard mixed meal ingestion is still scarce. In a study conducted by Meier at al. (2009), the authors reported that the absolute levels of glucose reached after an OGTT markedly exceed those reached under real-life conditions when compared to the results of a mixed meal tolerance test (8). Although this study only included non-pregnant patients, these results bring up the possibility that a mixed meal may be a more sensitive way to detect glucose intolerance even during pregnancy (11).
METHODOLOGY
For the writing of this article, we conducted a search between March 26th 2020 and April 28th 2020 in two electronic databases (Scopus and Pubmed) using the following Medical Subject Headings: Glucose Tolerance Test, Meal, Pregnancy and Diabetes, Gestational. Since the number of publications in this research area is limited, the research was not restricted in terms of publication date. In the end, 6 studies were included in this review.
Oral Glucose Tolerance Test
The diagnosis of GDM is usually established between 24 to 28 weeks of pregnancy with an OGTT, as insulin resistance increases during the second trimester (7, 12). This screening method has two different approaches: the one-step approach and the two-step approach (7). The one-step approach consists of a 75 g, 2-hour OGTT with the measurement of the fasting, 1-hour and 2-hour plasma glucose values (13). The two-step approach is based on a first screening with a Glucose Challenge Test (GCT) followed by a 100 g, 3h-OGTT. The GCT consists of a 50 g glucose load with the measurement of 1-hour plasma glucose values in a non-fasting state. If these values exceed 130 mg/dL or 140 mg/dL (depending on the institution where the test is executed), a 3h-OGTT is scheduled, consisting of a 100 g glucose load with the measurement of fasting, 1-hour, 2-hour and 3-hour glucose values (14).
However, to this day, there is still no consensus regarding what approach is more efficient, and consequently adopted worldwide. In 2010, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommended the universal screening of all pregnant women with the 75 g-OGTT to promote a more unified global guideline (15,16). This was accepted by The American Diabetes Association (ADA) and the World Health Organization (WHO) in 2011, but not by the American College of Obstetricians and Gynecologists (ACOG), which still stands by the two-step procedure (16). This divergence can be observed even in this review since both procedures are present within the different studies included.
After this, the WHO published a new guideline for the diagnosis of GDM using the IADSPSG criteria and the ADA decided to accept both the one-step and the two-step approach (13, 15, 17).
Certainly, no matter the procedure it can be an unpleasant experience given that some patients report side effects such as nausea, abdominal pain, headache and diarrhoea (10).
Criteria for the Diagnosis of Gestational Diabetes Mellitus
Different standards are used for the diagnosis of GDM, based on the adopted glucose load.
When it comes to the 75g-OGTT, the most accepted criteria worldwide are the IADSPG 2010/WHO 2013 criteria (18). These criteria assess the plasma glucose values in a fasting state, 1-hour and 2-hours after the administration of the oral glucose solution (13, 17).
As for the 100 g-OGTT, two sets of criteria are accepted: The National Diabetes Data Group 1979 criteria and the Carpenter and Coustan 1982 criteria, both based on the fasting, 1-hour, 2-hour and 3-hour measurement of plasma glucose values (19, 20).
The different diagnostic criteria for GDM are presented in Table 1.
Mixed Meal Tolerance Test as a Screening Method for Gestational Diabetes Mellitus
The mixed meal tolerance test dates to at least 1982 when Buss et al. compared both glucose and insulin levels during an OGTT and a MMTT in nine subjects who developed symptoms of hypoglycemia during an OGTT (21). This test has been studied as a possible alternative to the OGTT in the diagnosis of Diabetes Mellitus, including GDM, and is based in the ingestion of a meal containing carbohydrates, lipids and protein - a better representation of real-life diet patterns (8, 22). For writing this review, we conducted a search in two databases (Scopus and PubMed) for original articles comparing the OGTT to a MMTT. Studies were restricted to humans and articles written in English or Portuguese. We found studies comparing this method to a 50 g-GCT and others to a 75 g-OGTT. The constitution and nutritional composition of the meals used in these studies are presented in Table 2, but it should be noticed that some studies included did not give all the information concerning the macronutrients present in the meals tested. Also, to the best of our knowledge, there are no reports of studies comparing a MMTT to a 100 g-OGTT in pregnant women.
Mixed Meal Tolerance Test versus Glucose Challenge Test
Coustan et al. (1987) compared the plasma glucose values determined after a GCT with the plasma glucose values determined 1-hour after a 600 kcal MMTT in a group of 70 pregnant women between 25 and 33 weeks of gestation, 20 of which had already been diagnosed with GDM with the gold standard. For a cut-off point of 120 mg/dL, 16 of these subjects tested positive for gestational diabetes with the MMTT, presenting a sensitivity of 75% and a specificity of 94%. This study illustrates that the use of a standard mixed-nutrient meal as a GDM screening test may be as efficient as the use of a pure carbohydrate load, given that it has a predictable effect on plasma glucose, and more readily administered, particularly in circumstances of limited financial resources (23).
In a study conducted by Eslamian et al. (2007) 138 pregnant women between 24 and 28 weeks of gestation performed both GCT and MMTT. For a threshold of ≥ 130 mg/dL, 41 subjects screened positive with the GCT and 28 screened positive with the MMTT which had a sensitivity of 83.3% and a specificity of 85.9%. The authors concluded that a standard meal could be used as an alternative method for assessing carbohydrate intolerance in pregnancy with the same physiological response, probably better compliance and with lower costs (24).
Racusin et al. (2015) tested the use of candy twists as an alternative to the “glucola beverage”, using 20 pregnant women already screened positive for GDM by a GCT. For a cut-off of 130 mg/dL, the authors found
Table 1: Criteria for the diagnosis of Gestational Diabetes Mellitus
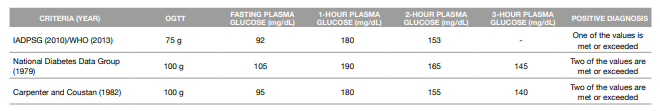
ADPSG: International Association of the Diabetes and Pregnancy Study Groups
OGTT: Oral Glucose Tolerance Test
WHO: World Health Organization
Table 2: Constitution and nutritional composition of the meals used in the meal tolerance tests
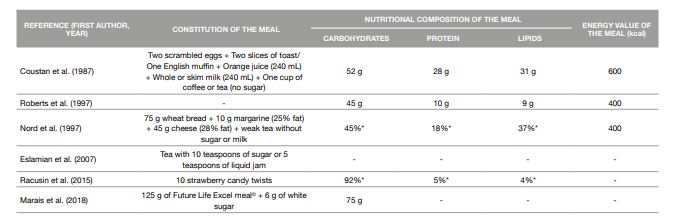
* percentage of the energy value of the meal
the sensitivity of the candy twists and GCT to be equivalent (100%), while the specificity, false referral rate and detection rate were improved in the candy twists test. The results obtained with the candy twists test allowed the authors to conclude that this alternative test could be as effective as the GCT and for a fraction of the price (0.97 USD for the MMTT vs. 3.41 USD for the CGT) (10).
Mixed Meal Tolerance Test versus Oral Glucose Tolerance Test (75g) Roberts et al. (1997) compared the results of a 75 g-OGTT and a 300 kcal MMTT performed one week apart in 102 non-diabetic pregnant women. There was no significant correlation between the two tests but in general, the glucose load caused a greater rise in plasma glucose compared to the mixed meal. However, they alert to the fact that the question of whether one test is more appropriate than the other can only be answered in a study with a large series of patients (25).
In a study conducted by Nord et al. (1997), although the main aim was to determine the postprandial substrate and hormone response to a 400 kcal mixed meal in healthy pregnant women and pregnant woman with GDM and not actually compare the OGTT and the MMTT as screening tests for GDM, the authors were still able to conclude that a MMTT may be more sensitive than a glucose load in detecting glucose intolerance (11).
Marais et al. (2018) compared a glucose test based on a standardized mixed meal to the 75 g-OGTT, while also comparing the use of venous and capillary glucose values in the diagnosis of GDM. When considering the venous 75 g-OGTT as the gold standard, the MMTT had a 25% sensibility, a 96% specificity, a 33% positive predictive value and a 94% negative predictive value. The authors reckon that the study population was too small to draw any conclusions about which test is more appropriate, but that the MMTT should be investigated further as an alternative test with merits (9).
CRITICAL ANALYSIS
The OGTT is the gold standard method for screening GDM, however, logistic and economic disadvantages in using a fast absorption carbohydrate solution make it advantageous to search for other screening tests (23). New methods of screening should be adequately sensitive and specific, yet easy to perform and cost-effective. The analysis of the studies included in this review has shown that the MMTT might be an option worth of further investigation since it better represents the real postprandial metabolic responses than the OGTT. Some of the factors that can contribute to this are the content of protein and lipids present in the mixed meal that delay the gastrointestinal glucose absorption (26). Also, gastric emptying is more rapid after the OGTT than after a meal, leading to a fast flow of glucose into the duodenum and the portal venous circulation in comparison with a meal that contains both proteins and lipids (27). A mixed meal is also generally more palatable and acceptable when compared with the OGTT, with none of the studies included in this article reporting side effects after the ingestion of the MMTT by the mother (28).
However, while reviewing the included studies, we identified some limitations that should be considered in future study designs. First, none of the studies assessed the acceptability of the alternative test by the subjects, which could express the need to reassess the constitution of the meal. In addition, none of the articles described the meal planning process, making it unclear to why those particular foods were chosen. Also, since not all the studies provided complete information regarding the nutritional composition of the meal, we were unable to make a systematic comparison between all the MMTTs. Another limitation present in all the studies included is regarding side effects, since there is no data available concerning the side effects for the baby. This is an aspect worth exploring in a future study. Lastly, some of the studies were conducted more than twenty years ago and for this reason, the methodologies used might be outdated - for example, Roberts and colleagues (25) used the 1980 WHO criteria to assess the pregnant women with impaired glucose tolerance, which is not the current WHO guideline.
We also observed some level of heterogeneity within the studies reviewed regarding the tests performed, the diagnostic criteria applied and the gestational age of the subjects, making comparisons among studies difficult.
The variations in both size and constitution of the meals used in the different MMTT could lead to different metabolic responses between the subjects (29, 30). When it comes to the OGTT, half of the studies compared the MMTT to a 75g glucose load and the other half to a GCT. Similarly, regarding the criteria for GDM, it varies depending on the glucose load used for the OGTT.
In future studies, some recommendations should be acknowledged concerning the meals used in the MMTT. For example, the caloric value of the meal should be calculated from the recommended daily caloric intake for the gestational age of the subjects at the time of the test and, since this value increases every trimester, the recruitment of subjects at the same gestational trimester should be considered (31).
Thus, the use of a standard mixed meal as the provocative test for diabetes screening might be as effective as the use of glucose load, if the standard mixed meal has a predictable effect on plasma glucose (23). Also, a standardized mixed meal would eliminate the confounding factor of variability in size and meal composition and would allow comparisons across different countries. However, for this to be a reality in the future, it is necessary to design more randomized clinical trials with a larger number of subjects than the ones presented here.
CONCLUSIONS
The MMTT could be a viable alternative to the OGTT, however, new studies should be conducted regarding this topic since the existing methodologies might be outdated. Also, this alternative test must be validated against the gold standard method in order to be considered for GDM screening. Besides, for the MMTT to be recognized universally, a standard meal should be designed to promote a unanimous guideline, as opposed to what occurs with the OGTT.














