Introduction
Sarcopenia may be simply described as a muscular disease, associated with poor health and socioeconomic outcomes. Despite not being an age specific disease, it is best known and studied in the geriatric setting. As our elders grow in number by the day, multiple international groups are emerging in order to address this problem.1-5
According to the European Working Group on Sarcopenia in Older People (EWGSOP), whenever a patient presents with reduced muscular strength (measured either by hand grip strength or the chair rise test), sarcopenia must be suspected. The diagnosis is then confirmed if there is evidence of diminished muscular quantity (determined, for example, by dual-energy X-ray absorptiometry or bioelectrical impedance analysis). Physical performance (gait speed, short physical performance battery, timed up and go test) can be additionally assessed in order to classify the severity of sarcopenia.1
The task force of the International Conference on Sarcopenia and Frailty Research (ICSFR), issued clinical practice guidelines for sarcopenia in 2018, suggesting that individuals who are 65 or older should be screened for sarcopenia at least annually.2 The SARC-F questionnaire is an easy and fast way of doing that same screening.1,2,6
Other definitions taking into account only the muscle mass is no longer considered to be adequate, since low muscle mass does not correlate as strongly as muscle strength to poor health outcomes.1,4
Interestingly, sarcopenia development may start as soon as early adulthood.3 It has a multifactorial etiology, being influenced by factors such as age, genetic background, nutritional status, chronic diseases, physical inactivity, inflammation.1,3 As previously mentioned, sarcopenia is associated with poor health outcomes, such as a superior risk of falls and therefore fractures; reduced cardiac, pulmonary and cognitive performance; inability to perform properly daily living activities with more dependence on others and eventually, increased mortality.1,5
Explaining sarcopenic obesity is challenging since an official definition does not exist, nor unanimous cut off points have been established in order to make its diagnosis.7,8 Sarcopenic obesity refers to the concomitant presence of low muscle strength/mass (sarcopenia) and excessive fat mass (obesity).1,7,8 The diagnosis workup proposed for sarcopenia is described above. For identifying excessive adiposity, a variety of criteria have been used (body mass index, percent body fat, waist circumference) and it is yet to be determined which one is better.7,8 Due to this heterogeneity in the diagnosis of sarcopenic obesity, the risk that it imposes in general health is also inconsistent, but growing evidence suggests a negative impact in quality of life, functionality, metabolism, risk of hospitalization and mortality.8
The best approach for reversing or at least improving sarcopenia and sarcopenic obesity is still not known. An exercise program associated with protein supplementation seems to be a promising solution.2 However, it is not an infrequent situation that elderlies do not have the proper conditions for entering an exercise program, or simply do not want to. Whenever this is the case, is there a place for nutritional supplementation alone? The authors proposed themselves to review the current knowledge about this topic.
Methods
For this narrative review of literature, the authors used three medical databases: pubMed, Web of Science and Cochrane. Whenever possible, the following filters were used: studies in humans, language (Portuguese or English), published in the last ten years (January 2011 to March 2021), study type (meta-analysis, systematic reviews, randomized controlled clinical trials and eventual post-hoc analysis of these clinical trials). In PubMed, the following query was used: (protein OR aminoacid OR multivitamins) AND (supplements OR supplementation) AND (sarcopenia OR sarcopenic obesity) AND (elderly OR very elderly OR older).
Other inclusion criteria are listed as following: studies with a significant sample of elderly people (above 60 years old), studies evaluating sarcopenia or sarcopenic obesity, studies evaluating oral nutritional supplements and their impact in sarcopenia or sarcopenic obesity.
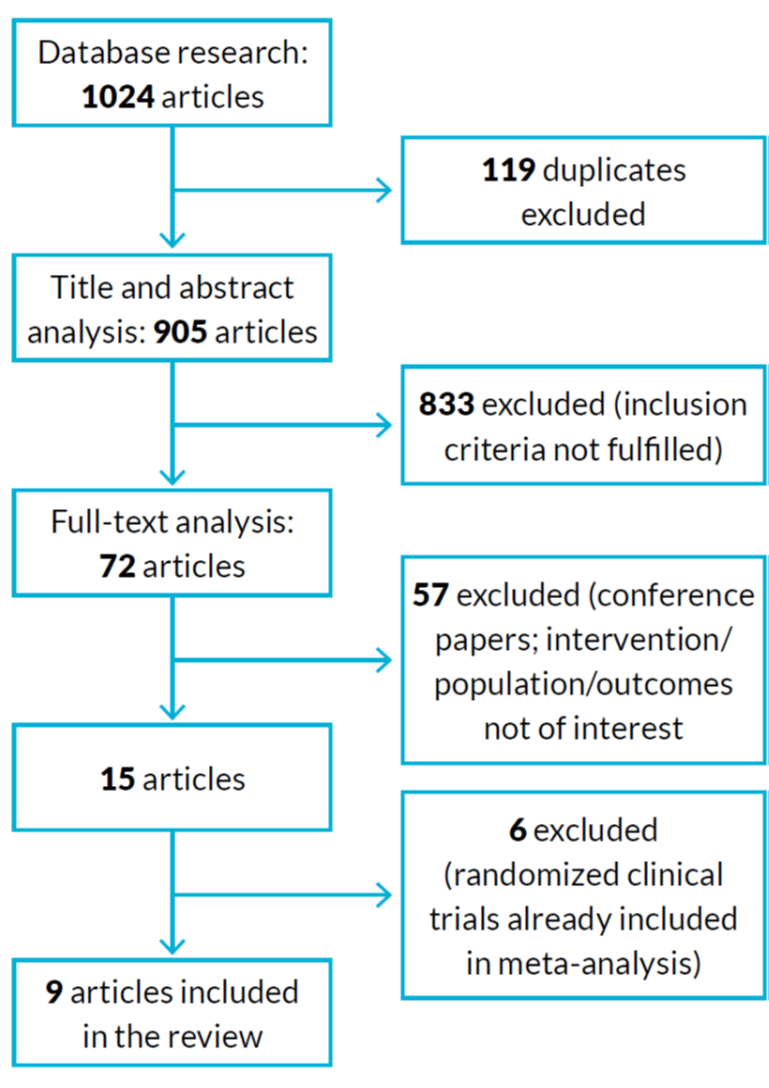
Studies were excluded if the nutritional supplement was given in association with other intervention (for example, exercise program), if the follow-up time was too short (less than four weeks) and, of course, if the inclusion criteria were absent. The primary outcome was an improvement in sarcopenic related parameters (such as hand grip strength, muscle mass, gait speed) with the use of nutritional supplements. The workflow of article selection is represented in figure 1.
Nutrition and sarcopenia/Sarcopenic obesity in the elderly
Yang et al meta-analysis aimed to determine the potential effects of oral supplementation with vitamin D3 in sarcopenia parameters, respectively handgrip strength.
A total of eight randomized clinical trials were included, with a sample size of 1334 patients, with a mean age of 65.2 years old. Intervention with vitamin D varied in dosage (100 to 20 000 IU/week) and follow-up time (3 to 12 months). There was no statistically significant difference between the treatment and placebo groups.
Subgroup analysis, with the exclusion of two studies involving extreme dosages of vitamin D (100 IU/day and 2800 IU/day), showed a beneficial tendency when supplementation with vitamin D was superior to 800 IU/day. There were some important limitations in this study identified by the authors, so these results must be used with caution.9
Martínez-Arnau et al systematic review included 23 articles, 13 of them being randomized clinical trials, five of them evaluating acute molecular effects of leucine (therefore not of interest to the present review). The main goal was to study leucine supplementation effects in different sarcopenic parameters. Leucine was administered alone or as a part of essential amino acids, whey/casein or other nutritional formulations. Dosages varied between 1.2 and 6 g per day. In nine of the included studies, there was concomitant supplementation with vitamin D (from 85 to 800 IU per day). The age of the included population varied between 61 and 87 years old and in six studies there were present other conditions besides sarcopenia, respectively chronic obstructive pulmonary disease (COPD), hepatic cirrhosis, type 2 diabetes and polymyalgia rheumatica. According to the authors, 16 of the studies evaluated the variation in muscle strength with supplementation: six of them reported an improvement and one a disimprovement. Additional elements in the supplementation (such as vitamin D) or the form of administration (essential amino acids, whey protein or casein) did not have a clear impact in this parameter.
Again, 16 studies analysed the effect of supplementation in lean body mass, 10 of them reporting a beneficial effect (most of them, however, also had vitamin D). Six studies reported the effects on physical performance, with three of them having beneficial effects with leucine (with or without vitamin D, as essential amino acids or whey protein). In general, the different formulations were well tolerated. The impact of leucine alone in the population with other disorders besides sarcopenia was much weaker, nearly absent (beneficial effect in muscle strength in COPD). Interestingly, some studies mentioned an improvement in other areas when leucine was given, such as cognitive function and depressive symptoms.
It is important to keep in mind that in many studies, leucine was not given alone, so we cannot know for sure the exact benefit that actually is related to leucine.10
Martínez-Arnau et al then proceeded to elaborate a double-blind randomized clinical trial to evaluate the variation of sarcopenic, respiratory and blood parameters with leucine only supplementation. The EWGSOP criteria were used. They enlisted a group of 50 participants from nursing homes in Spain, aged 65 or older, to either receive daily supplementation of L-leucine (6 g) or placebo (6 g of lactose). These were given in a glass of water/juice in the morning (3 g) and afternoon (3 g), for a period of 13 weeks. Eight patients (five from the treatment group and three from the placebo group) did not complete the intervention. Baseline characteristics were similar between groups, with exception of muscle max index (muscle mass/height2), which was superior in the control group. With intervention, there was a significant difference in walking time compared to baseline, with the placebo group taking the longest to complete the determined distance. Handgrip strength, muscle and fat mass indexes, calf and arm perimeters did not differ.
Maximum expiratory pressure at mouth (a parameter related to respiratory muscle strength) significantly reduced in the control group. Other pulmonary and blood parameters did not have important differences.11
Another systematic review and meta-analysis dedicated to evaluating the effects of leucine formulations in the elderly is the one from Komar et al. Sixteen studies were included (randomized clinical trials and cross-over), four in common with Martínez-Arnau et al, representing a total of 999 patients, aged 65 or older. Dosages of leucine varied between 2 and 7.8 g per day and were administered as a part of essential amino acids, whey/casein protein, ricotta cheese or protein energy drinks. Serious adverse effects were not reported.
Only six of the studies had sarcopenic individuals (n=397), and subgroup analysis was made in order to understand supplementation effects in this particular setting. Body weight and lean body mass increase was significant in treatment groups, particularly if sarcopenia was present. Body mass index also significantly improved after supplementation, however without significant differences between healthy and sarcopenic patients. On the contrary, fat mass, percentual body fat and muscle strength were not significantly altered with leucine supplementation. Similarly, to Martínez-Arnau et al systematic review, since leucine was mainly given with other additional elements, it’s hard to establish the benefits of this amino acid alone.12
The Provide Study, a double-blind randomized clinical trial included in the previously mentioned Martinez-Arnau et al systematic review, needs to be specially mentioned in order to facilitate the comprehension of two other articles included in our review. 380 sarcopenic patients, aged 65 or older, were distributed between two groups, either to receive the active treatment (whey protein, 20 g; leucine, 3 g; essential amino acids, 11 g; carbohydrates, 9 g; fat, 3 g; vitamin D, 800 IU; other vitamins, minerals - such as calcium, 500 mg - and fibers whose quantities may be consulted in supplemental material of the original article) or a placebo formulation (carbohydrates, 31 g; fat, 3 g; and some minerals - sodium, potassium and chloride), twice a day, for 13 weeks. Forty elders from the treatment group and 38 from the control group, did not complete the study. Handgrip strength, gait speed, balance tests and, therefore, the short physical performance battery did not differ significantly between groups after the intervention. There was a significant beneficial effect in appendicular muscle mass and chair stand test capability in the treatment group.13
A posterior analysis from Hill et al, evaluated the effects of supplementation on different bone parameters. In the treatment group, there was a significant increase in vitamin D, insulin-like growth factor 1 and to a lesser extent, bone mineral density and calcium. Additionally, parathormone was effectively suppressed in this group and a marker of bone reabsorption significantly reduced (despite having reduced in both groups, it was most prominent in the treatment group). Bone formation parameters did not differ between groups.14 Since chronic inflammation seems to be present in sarcopenia, Liberman et al studied the effects of the nutrition formulation used in the Provide Study in multiple inflammatory parameters. There was an overall increase in the levels of interleukins (IL) in both groups during the follow-up period, with exception for IL-8 (significant decrease in the treatment group). Also, for IL-6, the increase was mitigated with supplementation. These results might indicate a potential beneficial effect of supplementation in inflammatory parameters associated with sarcopenia.15
Cramer et al also studied protein effects in sarcopenia in a randomized, double blind, clinical trial. Participants (n=330) had both malnutrition and sarcopenia, were aged 65 years old or more, and were selected from eight different countries from Europe and North America.
They could either receive the control supplement (protein 14 g; fat 11 g; carbohydrates 44 g; vitamin D 147 IU; and other elements) or the experimental one (protein 20 g; fat 11 g; carbohydrates 36 g; vitamin D 499 IU; leucine metabolite, methylbutyrate 1.5 g; and other elements), two times a day, for 24 weeks. Both formulations had active ingredients, with a potential treatment effect, so there was not an actual control group, which is one of the downsides of the present study. Furthermore, all participants, independently of the study arm, had a minimum consumption of 0.8 g/kg of protein in their daily diet. In each group, participants were further characterized into severe sarcopenia (both gait speed and grip strength impaired) or mild to moderate sarcopenia (just one of the previous impaired). Leg strength and muscle quality improved in both groups, without significant differences between them. Subgroup analysis, for mild to moderate sarcopenic individuals with normal grip strength, showed a superior effect with the experimental formulation, significant at 12 weeks, but not at 24 weeks for both these parameters. Grip strength, gait speed, body mass index, body weight, fat mass increased in both groups, without differences between the two formulas. Leg muscle mass did not change. Therefore, this study alerts us to the fact that sarcopenic elders might have different responses to nutrition formulations according to the severity of their clinical situation. Similarly, to other studies, since the supplements had a lot of components, it is not possible to determine eventual benefits of individual substances.16
EWGSOP/IWGS systematic review from 2014, evaluated existing interventions applicable in the treatment of sarcopenia, one of them being nutrition. Twelve articles were included about this matter, 11 involving community patients (62 to 90 years old) and one with institutionalized elders (mean age 83 years). Most studies, however, had small sample sizes (minimum of 14 and maximum of 155), limiting the validity of results. Five articles were related to protein supplementation, but only in one of them was protein used alone. Overall, there were not significant nor consistent results regarding this kind of supplementation. Two studies used essential amino acids, leucine being of notice (2.5 and 2.8 g). One of them included healthy individuals only. In the latter, muscle mass significantly improved with intervention. This did not happen in the second study, with only physical performance having a positive response to supplementation. In four studies the chosen supplementation had β-hydroxy β-methylbutyric acid, either alone (two studies) or in association with other essential amino acids (one study) or exercise (one study). Once more, the results were not consistent, although there might be some beneficial effects on muscle mass and function. The one study with linolenic acid did not show significant results.17
Finally, when it comes to sarcopenic obesity, information is sparse in comparison. One meta-analysis was included, with five out of 15 studies being related to nutrition.
The mean age in these five studies varied between 55 and 81 years old and again sample sizes were small (18 to 139 individuals). Three studies compared supplementation (isoflavones, protein and essential amino acids) versus placebo and two studies did not resort to a particular supplement, but instead compared two low calorie diets, differing in protein quantity (normal or high). Overall, nutrition reduced fat mass, but did not ameliorate muscle mass or grip strength. Subgroup analysis showed this to be true specifically for low calorie high protein diet. Supplements did not have a positive impact in any of these parameters, including fat mass.18
Discussion
This review aimed to unravel the existing knowledge regarding nutrition as a therapeutic option in the treatment of sarcopenia and sarcopenic obesity. Clearly, the interest and research about this matter is rapidly spiking, as it should be when we take into account the increasingly geriatric population in our world.
According to EWGSOP/IWGS, sarcopenia prevalence varies significantly across different populations, but it may be as high as 33% in some contexts.17 Due to the absence of an official definition to sarcopenic obesity, its prevalence is harder to establish.
There were multiple limitations identified in the information gathered for the current review. The definitions and cut-off points used to identify sarcopenic individuals varied importantly among studies. There were occasions where sarcopenia and frailty were used as synonyms, which is not the case. Frequently, oral supplements had multiple active components, with different quantities, making it impossible to establish the true effect of a particular one. There was heterogeneity in population baseline characteristics, follow-up time and outcomes of interest.
Additionally, sample sizes were overall quite small.
The previously mentioned ICSFR guidelines draw some recommendations about sarcopenia’s approach. Protein supplementation/high protein diet should be considered in the management of sarcopenia, although the level of evidence is low (as opposed to resistance exercise, with a moderate level of evidence and a strong recommendation).
No ideal quantities are, however, mentioned. They found insufficient data about using vitamin D alone, as such, it is not advised.2 The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends a daily protein consumption of at least 1.0 g per kg of body weight in elder people.19 Again, the ideal amount in sarcopenia needs to be further assessed.
Leucine (or leucine metabolite, methylbutyrate) enriched protein supplements may constitute an interesting option.
However, future studies (ideally large randomized controlled clinical trials), with consistent and well-defined criteria are in need in order to confirm this hypothesis.
EWGSOP criteria have a sensitivity and specificity superior to 80% for diagnosing sarcopenia.2 Furthermore, it would be useful to study leucine only supplementation, in order to finally establish its actual benefits. The same goes for vitamin D or any other supplement. As for sarcopenic obesity, an official definition is yet to be determined and eventual benefits of nutritional supplements are less clear.
Finally, another important aspect to have in mind is what outcomes are actually clinically relevant. As proposed by Komar et al, for sarcopenic elders it might be enough to stabilize muscle parameters, and prevent a further decrease, instead of increasing them.12