Introduction
Endometriosis is an inflammatory disease characterized by lesions of endometrial-like tissue outside the uterus. 1 Lesions are frequently categorized according to the location and depth of infiltration into the tissues, and include superficial peritoneal lesions, deep infiltrating disease, ovarian endometriomas, and extra-pelvic lesions.
It affects 10%-12% of women in their reproductive years, and can have considerable adverse impacts on quality of life, including an increased risk of infertility.
The pathogenesis of endometriosis combines both genetic and environmental influences. Many aspects of the etiology of the disease are still obscure, but different studies (Familial Studies, Linkage Analysis and Genome-Wide Association Studies) (2-4 have given us information regarding the physiopathology of endometriosis through ample evidence, associating cellular procedures with disease development.
Although endometriosis is proven to be a benign disorder, clinical, histopathologic, epidemiologic and molecular data suggest that it does share common characteristics with cancer, including inflammatory processes, resistance to apoptosis, angiogenesis and invasion of other tissues.
In 1925, Sampson 5 was the first to report a relationship between endometriosis and ovarian cancer and proposed a theory of malignant transformation of endometriosis. Czernobilsky and Morris described later an intermediate stage in the malignant transformation called atypical endometriosis characterized by cytologic atypia and architectural atypia or hyperplasia.
According to a comprehensive review 6 on the frequency of EAOC, the most frequent pathologic ovarian cancer subtypes were clear cell carcinoma (35% in 390 cases) and endometrial carcinoma (27% in 648 cases), followed by serous carcinoma (5% in 1372 cases) and mucinous carcinoma (4% in 614 cases).
In terms of information and reassurance to the patients with endometriosis we should emphasize that the disease has a small increased risk of developing ovarian cancer compared to the general population.
In the general population, it is estimated that 1.3% of the women will develop ovarian cancer in their lifetime. Considering the relative risk calculated from meta-analyses of endometriosis and ovarian cancer, the lifetime risk of ovarian cancer among women with endometriosis is 1.9%. 7,8 This small increase in absolute risk should thus reassure women with endometriosis that their lifetime ovarian cancer risk is quite low and, in absolute risk scales, is only negligibly different from women without endometriosis.
The World Endometriosis Society (WES) (9 and the European Society of Human Reproduction and Embryology (ESHRE) guidelines 10 also stated that the relative and absolute risk of ovarian cancer in women with endometriosis is very low and routine screening for ovarian cancer was not recommended.
Cases Report
In 2021 we came across with five different cases that somehow met the criteria proposed by Sampson for the endometriosis-ovarian cancer association: 1) clear evidence of endometriosis found close to the tumor, 2) histopathologic appearance consistent with that of endometriosis, and 3) no other primary tumor site found. Later on, Scott added a fourth criterion: morphological continuity of transition from benign to malignant tissue in endometriosis. (11 Furthermore, the cases occurred in young women, with a previous diagnosis of endometriosis, and some presenting with infertility, which are also some clinical features associated with EAOC.
Interestingly, we had three cases with the most frequent tumors linked to endometriosis (clear-cells, endometrial and serous), one case of carcinosarcoma and one case of atypical endometriosis.
Scientific articles with relevance for discussion of this topic were searched using PubMed and the criteria used for the inclusion of publications was that they had in the title, or keywords, or explicit in the abstract that their content was related to the association between ovarian cancer and endometriosis and preferentially published in the last decade.
Case 1: Atypical endometriosis
A 40-year-old Caucasian patient with past gynecological medical records of primary infertility was referred to consultation due to severe dysmenorrhea unresponsive to medical therapy for three years.
She had a voluntary interruption of pregnancy in her youth, and two years ago she underwent two unsuccessful IVFs (in-vitro fertilization) treatments. Last year she got pregnant spontaneously but she had a miscarriage at 8 weeks of pregnancy.
Transvaginal ultrasound revealed an adnexal mass in the left ovary compatible with an endometrioma.
Patient underwent a laparoscopic left ovary cystectomy without complications. Anatomopathological examination revealed fragments of ovarian parenchyma with cystic lesions and micropapillary areas of architectural complexity with cytological atypia. Atypical endometriosis lesions (Fig. 1).
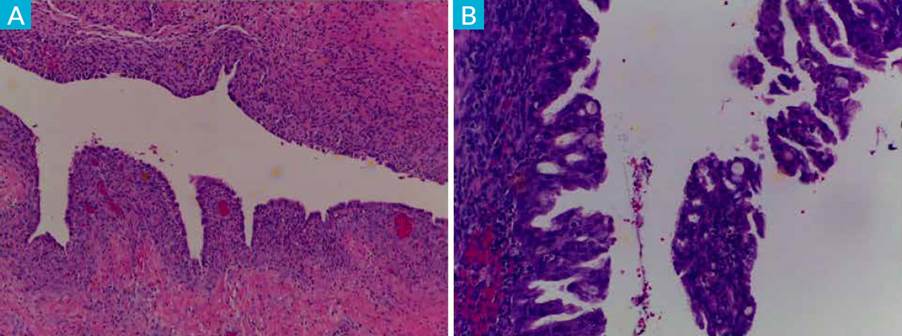
Figure 1 Endometriosis lesions: A) with presence of endometrial-like stroma and epithelium (HE, 4x). B) associated with epithelial proliferation with architectural complexity and cytological atypia - hyperplasia with atypia (HE, 10x).
Atypical endometriosis may be a precursor lesion of EAOCs but is not systematically found in all cases. AE refers to two different histologic findings: cellular or cytologic atypia, and architectural atypia, commonly referred to as hyperplasia (resembling endometrial hyperplasia of the endometrium). The diagnosis of endometriosis with architectural atypia, as found in our case report, is important because patients with this type of atypical endometriosis may be at a higher risk of developing EAOC. (12
The case was presented to our gynecological tumor board and, as the patient wanted to preserve her fertility, and since there are no clinical guidelines regarding postoperative follow-up and no scientific evidence indicating that radical surgery (total hysterectomy with bilateral salpingo-oophorectomy) could benefit the patient, she was encouraged to prioritize pregnancy and to have a prudent and long-term follow-up.
Case 2: Clear-cell Carcinoma
A 46-years-old Caucasian woman with a previous history of chronic abdominal pain and infertility in the context of endometriosis. Familiar history was negative for cancer, namely breast and ovarian cancer.
Three years earlier, the patient underwent laparoscopic surgery due to deep endometriosis. Excision of multiple deep infiltrating endometriotic foci and a partial bladder cystectomy was performed. Anatomopathological examination confirmed endometriosis.
She had two pregnancies. The first one achieved after IVF treatment and a spontaneous second pregnancy with delivery 6 months before present history. Currently she is under oral combined contraception.
The patient was admitted in ER with severe abdominal pain and abdominal enlargement with no other sign or symptom. Hemogram, renal and liver function tests were normal. An abdominal and pelvic computed tomography (CT) was performed and revealed moderate ascites, multiple peritoneal implants, including subcapsular liver implants, the greatest with 4.5 cm, and bilateral multiloculated cystic adnexal lesions with 50 mm on the left and 26 mm on the right were identified (Fig. 2). External iliac lymph nodes were suspected of metastatic disease.
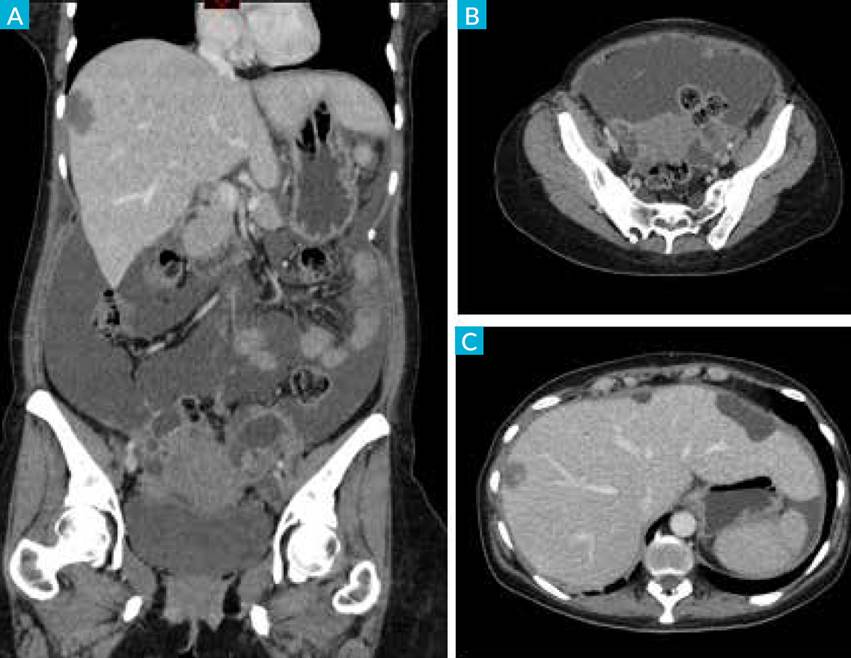
Figure 2 CT-scan with intravenous contrast in coronal (A) and axial (B, C) planes, showing ascites in the abdominal and pelvic cavity with peritoneal micronodularity and implant in the serosa of the liver (right lobe). Adnexal cystic lesions with solid component.
A paracentesis was performed and the cytological evaluation of the ascitic fluid revealed atypical epithelial cells.
A diagnostic laparoscopy revealed an unresectable disease. Anatomopathological results of peritoneal biopsy were positive for metastasis of a clear cell carcinoma with a probable origin in the ovary, CK7, PAX8, NapsinA, Racemase P540S/AMARC positive, p53 positive, WT1 negative.
She started neoadjuvant chemotherapy with carboplatin AUC 6 and paclitaxel 175 mg/m2.
Evaluation after the third cycle of chemo in order to assess the possibility for interval cytoreductive surgery was disappointing. CT scan revealed progression of the disease under therapy with sudden and progressive worsening of the general status of the patient, who died 6 months after the diagnosis.
Ovarian clear-cell cancer (OCCC) patients are easier to be resistant to chemotherapy, have a worse prognosis, and are usually difficult to treat. (13
As OCCC are often associated with early occurring resistance to platinum-based chemotherapy, the rationale is to identify targetable molecular key-mechanisms that provide potential new therapeutic opportunities.
Besides target therapies and immunotherapy, the assessment of ARID1A (tumor suppressor gene) in OCCC patients may also be helpful in the choice of chemotherapy. Gemcitabine appears to be the most effective chemotherapy agent in platinum-resistant OCCC with response rate estimated to 66% (n=12) in a sub-group analysis of the MITO-9 study (149). This was confirmed in another small retrospective cohort from Japan (n=7) that showed that three ARID1A-deficient OCCC patients had a significantly longer progression-free survival with gemcitabine compared to four OCCC patients. (14
Case 3: Endometrial Carcinoma
A 44-year-old Venezuelan nulliparous woman, with primary infertility and a surgical past of laparoscopic endometriosis surgery in 2008 in Venezuela. A bilateral cystectomy was performed.
She was admitted to the ER due to abdominal bloating and pain and referring abdominal enlargement. A palpable mass was found on pelvic examination and ultrasonography outlined a large 10 cm solid-cystic lesion in the left adnexa with fine echogenic particles, thick septation, thick wall and a solid component.
The patient was asymptomatic and did not use any contraception because her sexual partner has azoospermia. The couple had four ART (assisted reproductive technology) treatments without success, and she had already given-up motherhood.
Two years ago, she had a transvaginal ultrasound (TUS) revealing bilateral ovarian cysts 3-4 cm that were suggestive of endometriomas. Due to the COVID-19 pandemia she did not perform the prescribed control TUS.
Further investigation was done at the ambulatory clinic.
Blood tests and CA-125 were all within the normal limits. Tumor marker CA-125 may be helpful, but its usefulness in the diagnosis of early EAOC is limited due to lack of specificity. Indeed, moderate elevations of CA-125 often occur in women with benign conditions (endometriosis, adenomyosis, leiomyomas, inflammatory pelvic disease) without any evidence of ovarian cancer. (14
MRI revealed a left adnexal solid-cystic lesion measuring 11 cm showing thick and irregular walls and thick septa with mural nodules, one of them measuring 4.3 cm (Fig. 3-A). These findings were suspicious for ovarian malignancy (endometrioma malignization?).
Another cystic mass of the right ovary with 5 cm suggestive of endometrioma and some lesions suggestive of deep infiltrating endometriosis of posterior compartment 15) were described. There was no ascitic fluid. Compression of the left common iliac vein by the right common iliac artery (May-Thurner syndrome) (16 was found. CT-Scan reported a slight bilateral ureterohydronephrosis due to probable extrinsic compression by the lesion on left and to ureteral involvement by deep endometriosis on right. There were no criteria for pelvic, abdominal or thoracic adenopathy. No metastatic disease was found on the thorax or abdomen.
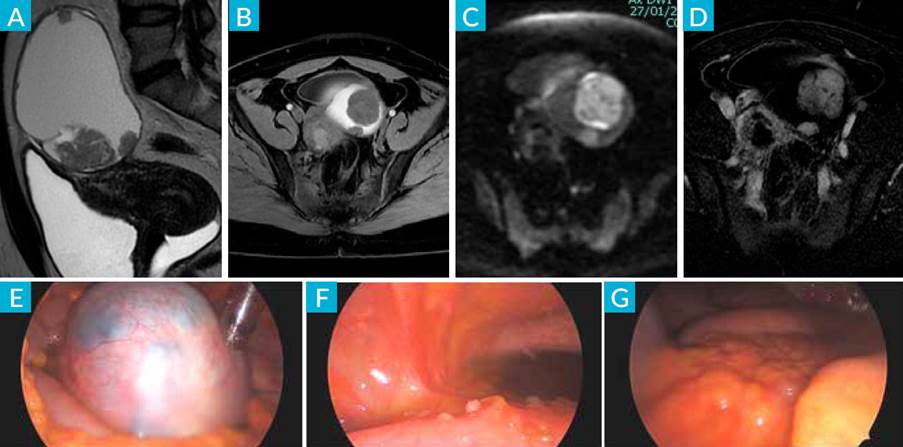
Figure 3 MRI: sagittal T2 (A), axial T1 fatsat (B), DWI b1000 (C) and subtracted image after ev contrast injection (D), showing left adnexal cystic lesion with hematic content (*) and solid component (arrow) with contrast uptake and diffusion restriction, suggesting malignancy. E) laparoscopic view of left adnexal mass: F) implants affecting sigmoid serosa: G) omental cake.
After stent insertion, she underwent laparoscopic surgery (Fig.s 3-B, C and D) and then a debulking surgery by median laparotomy, based on a peritoneal implant frozen-section diagnosis of malignancy. Intraoperatively a frozen pelvis and huge bilateral adnexal masses were found, and a sub-optimal cytoreductive surgery with a bilateral salpingo-oophorectomy, infra-colic omentectomy, pelvic and periaortic lymph node sampling and excision of visible abdominal tumor lesions was done. Hysterectomy was not achieved because the uterus was completely submerged with the bladder and the sigmoid within a tumoral process which extended to the pelvic side wall, occluding the pelvis.
The postoperative period was complicated by deep vein thrombosis, probably due to the May-Thurner syndrome.
Final pathology reported bilateral endometrioid carcinoma G3 of the ovaries pT3cN1aM0 (FIGO IIIC), with pelvic positive lymph nodes.
She recently began platinum/taxane based systemic chemotherapy, and a second-look cytoreductive surgery is planned.
The risk of EAOC is higher in patients with a long history of endometriosis, big endometriomas (>9 cm), and advanced stage endometriosis. These patients should be under close observation. (14,17 In this case we had a fourteen years old diagnosis of endometriosis and an adnexal mass with 11 cm which fits within risk data. On the other hand, significant differences between early stages (stages I and II, 73.8% vs 41.8%, p<0.00001) and low histological grades (33.3% vs 19.3%, p<0.001) were found between EAOC and non-EAOC patients, suggesting a significant association of endometriosis with low tumor grades and early-stage tumors. These might explain the better prognosis of EAOC patients, in that early-stage cancer might be a driver for better prognosis. (18 This earlier diagnosis may be also due to the more pronounced symptoms seen in patients with endometriosis compared with the few symptoms found in patients with non-EAOC, which leads them to seek medical attention earlier and more often and require more frequent gynecologic imaging tests.
Unfortunately, our patient did not match this last data, and the diagnosis was an advanced stage FIGO IIIC, histological grade 3 endometrioid carcinoma, therefore with a worst prognosis.
A separate note about the impact of the COVID-19 pandemic on women's health, which has happened in this case, was an additional risk factor, because it delayed for almost 2 years the medical investigation and potentially an early diagnosis of the disease which could result in a better outcome.
We believe that this impact will be felt more frequently as more patients will appear with diseases diagnosed in more advanced stages, or with ongoing diseases but poorly monitored.
Case 4: High-grade Serous Carcinoma
A 34-years nulliparous woman trying to get pregnant, without success. She uses no oral contraception, and she complains of frequent menstrual hemorrhage (polymenorrhea), dysmenorrhea (VAS score 7/10) and dyspareunia (VAS score 8/10).
On pelvic examination a painful uterosacral ligament nodule was palpated, and a systematic sonographic approach as defined by International Deep Endometriosis Analysis (IDEA) consensus (Guerriero et al 2016) (19 were suggestive of deep infiltrating endometriosis (DIE) of the posterior compartment.
On magnetic resonance imaging (MRI) (Fig. 4), both ovaries were normal with the left ovary adherent to the uterine wall. No evidence of endometriomas bilaterally. Retrocervical thickening, with involvement of the uterine torus, with a nodular thickening of the rectovaginal septum was noted. A transmural nodule of the anterior wall of the rectum, 8 cm apart the ano-rectal junction and a band thickening of the uterosacral ligaments with greater expression on the right with 16 mm were also noted.
Transvaginal ultrasound, because of its non-invasiveness, availability, and cost-effectiveness, is considered by many endometriosis experts as the best first line method for assessment of DIE. MRI is also considered a highly accurate imaging modality in the evaluation of DIE, particularly when involvement of the rectum, ureters and nerve roots is suspected; these areas are highly important for both the patient and the surgeon and may be poorly visualized even with laparoscopy. (15
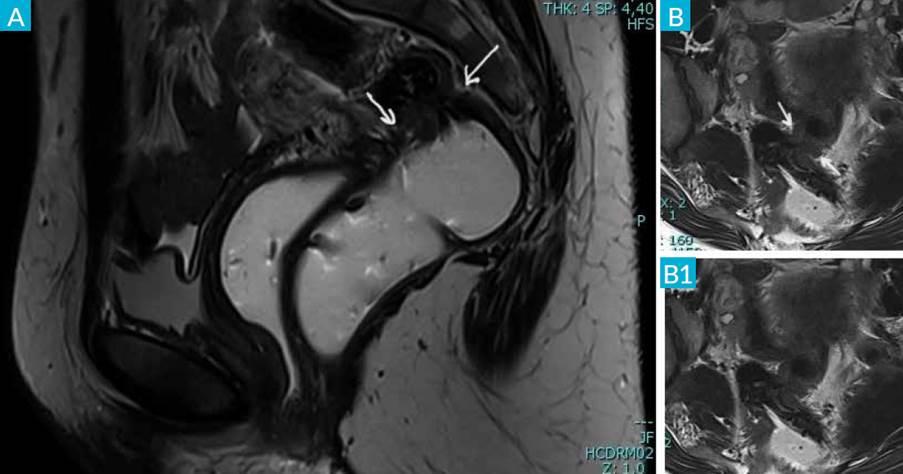
Figure 4 CT-scan: sagittal T2 (A) and axial T2 (B and B1) sequences with gel filling of the vagina and rectum show nodular thickening in the anterior wall of the rectum, with luminal reduction (white arrow). There is a slight retraction of the posterior vaginal fornix.
The patient denies rectal bleeding or dyschezia and a colonoscopy revealed no mucosal involvement.
A multidisciplinary laparoscopic surgery team performed an anterior discoid resection of the rectum and excision of several suspected deep endometriotic pelvic foci.
The surgical treatment of DIE is a complex procedure and requires wide knowledge of the disease process and should be managed in a multidisciplinary team led by a specialist gynecologist. (20
Histopathological examination revealed high-grade serous carcinoma contiguous with rectal endometriosis (Fig. 5) and multiple high-grade serous carcinoma implants.
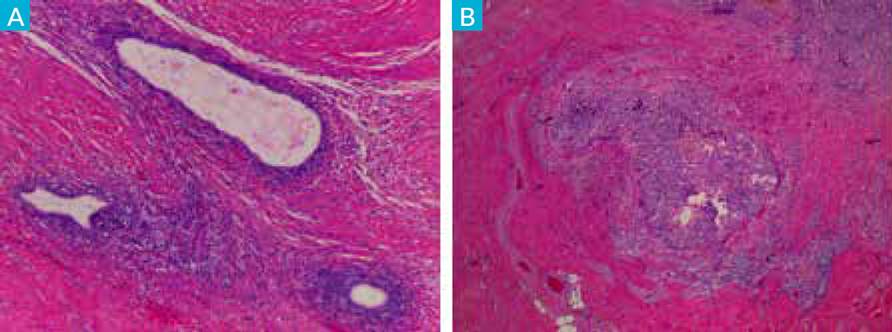
Figure 5 A) Intestinal muscle wall with endometriosis lesions (HE, 10x): B) Intestinal muscle wall partially occupied by high-grade serous carcinoma of tubo-ovarian origin (HE, 4x).
As mentioned before, several studies suggested that endometriosis increases the risk of some malignant tumors, 80% of which occur in the ovary. However, extragonadal endometriosis accounts for 5.7% of all malignant transformations of endometriosis, 4.3% of which occur in the rectovaginal septum, and another 4.3% in the colorectum. Our case seems similar to the Li Song et al (2019) (21 reported case of high-grade serous carcinoma resulting from rectal endometriosis and complicated with ovarian cancer.
Unfortunately, we lost track of this patient, because for economic reasons the patient had to continue further therapy in another Institution.
Case 5: Carcinosarcoma
A 53-year-old Dutch woman, 2 gesta 2 parous, was referred to us with a histology of high-grade serous carcinoma of the ovary.
Previously, two transvaginal ultrasounds revealed an ovarian cyst with 5 cm compatible with endometrioma, that grew to 10 cm within a 2-month period. She was being prepared for elective surgery, when she was admitted to the ER of a private clinic, with an acute abdomen and a suspected ovarian cyst torsion. An emergency laparoscopic unilateral ooforo-salpingectomy was performed, and the histology revealed a high-grade serous carcinoma with tumor on the fragmented capsule of the ovary.
After discussion on our gynecologic tumor board, a cytoreductive surgery was performed with total hysterectomy and unilateral salpingo-oophorectomy, pelvic and lombo-aortic lymphadenectomy, omental resection, pelvic peritonectomy (due to the presence of implants in the posterior cul-de-sac), and multiple biopsies including the previous laparoscopic entry ports.
No visible disease (CR0) at the end of surgery.
Histopathological examination revealed a carcinosarcoma in the right tube with high-grade serous carcinoma on the epithelial component and chondrosarcoma on the mesenchymal element of the tumor (Fig. 6). Uterine adenomyosis and several foci of endometriosis were found on ovary, cervix, parametrium and uterine serosa.
High-grade serous tumor was present on the omentum, but it showed no lymph node metastasis.
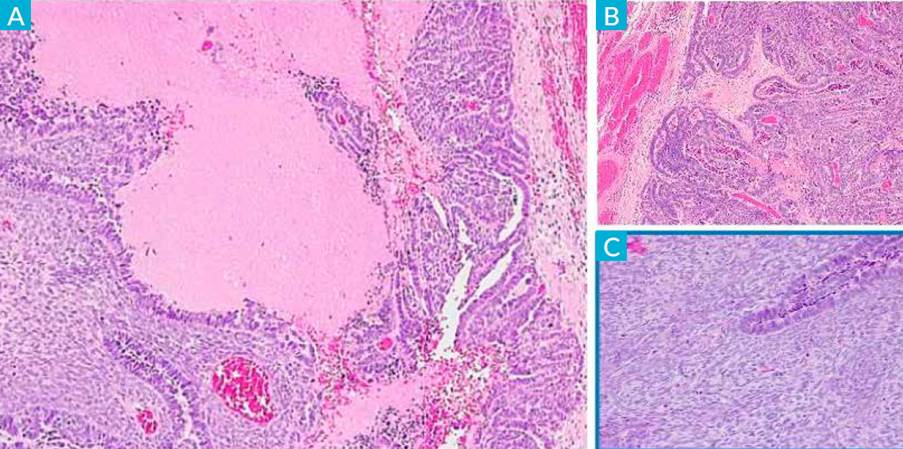
Figure 6 A) Fallopian tube carcinosarcoma (HE4x): B) epithelial component (HE20x): C) stromal component (HE20x).
Carcinosarcoma arising from endometriosis is exceedingly rare. (22,23 As mentioned before, most malignant ovarian tumors arising from endometriosis are clear cell carcinoma or endometrioid adenocarcinoma. Few reports exist of sarcoma associated with endometriosis, and even fewer exist of multiple types of malignancies occurring simultaneously.
A recent Phase III trial (GOG trial 0261) compared paclitaxel/carboplatin to paclitaxel/ifosfamide. The study found that paclitaxel/carboplatin was not inferior to the regimen containing ifosfamide. Additionally, paclitaxel/carboplatin was associated with prolonged progression-free survival in patients with recurrent carcinosarcoma of the uterus or the ovary (16 months vs 12 months). (24
For geographical reasons, the patient preferred to return to her area of residence to undergo the chemotherapy treatments that were proposed, and booked a new appointment after 6 cycles of platinum-based therapy, to continue the follow-up.
Though cytoreductive surgery has a major impact on morbidity and survival, recurrence of cancer (particularly with gynecological tumors) is encountered frequently, therefore, surveillance and early detection of relapse is mandatory.
Discussion
While endometriosis is a common disease, the overall risk of an endometriosis-associated ovarian cancer remains low.
Analysis of germline genetic risk association data demonstrated that EAOCs shared many genetic mutations with associated concurrent endometriosis lesions, including mutations in ARID1A (AT-rich interactive domain-containing protein 1A).
ARID1A is a tumor suppressor gene that regulates important cellular functions (proliferation and genomic stability) therefore, it was thought that it might play a role in the transformation of endometriosis to cancer. (25
PIK3CA (phosphatidylinositol-4,5-bisphosphate3-kinase) is a protein coding gene and has long been described as an oncogene. Shared mutations in PIK3CA were also detected between endometriosis and clear-cell lesions. Several studies investigated the correlation between PIK3CA mutations and the presence of AE. (26 All of these studies reported concordant mutations in PIK3CA either in cancer or in contiguous AE.
The description of all genetic mutations that establish the association between endometriosis and cancer is beyond the scope of this review, although they support the finding that a high proportion of clear-cells and endometrial ovarian cancers are associated with endometriosis (20%-50%).
There are two important issues concerning AE: first, the possibility of its coexistence with cancer, and second determining how to conduct a follow-up of those patients. Tanase et al27 emphasized the importance of considering the possibility of malignant change, and strongly recommended carefully following patients in cases when AE is observed.
There are neither clinical guidelines nor scientific evidence indicating how to manage cases diagnosed as AE, especially at a younger age, and so these patients need to be followed for long-term periods. (28
EAOC is presented with some specific clinical features that distinguish it from non-endometriosis-associated ovarian cancers: it affects younger patients, shows lower CA-125 levels, and it has a better prognosis because usually is diagnosed in earlier stages. (29) Women with endometriosis are more aware of physical discomfort, so they are more likely to see a doctor earlier, and may require more frequent gynecological ultrasound. This may explain the reason why OC patients with endometriosis were usually diagnosed at an early stage.
The following clinical groups are at an increased risk of developing EAOC: patients aged > 45-50 years; nulliparous patients; long term history of endometriosis; diagnosis in postmenopause; endometriomas ≥ 9 cm; hyperestrogenism. (30
Closer monitoring is advised for this patient population.
Some preventive measures can be taken to reduce the risk of developing ovarian cancer. (25 Studies on the relationship between oral contraceptives and ovarian cancer indicate reduced ovarian cancer risk with long-term use of oral contraceptives. Similarly, tubal ligation showed a reduction of risk of almost 50%. Two large studies (Nurses' Health Study and Nurses' Health Study II) have focused on the effects of tubal ligation in decreasing the risk of ovarian cancer. Tubal ligation has a protective effect for clear-cell and endometrioid carcinoma, which is believed to be related to the prevention of retrograde menstruation, ovarian seeding by endometrial cells, and inflammation. Furthermore, for those women looking for permanent contraception, opportunistic salpingectomy also has the potential to reduce their risk of serous ovarian cancers and should be considered.
The ovarian cancer risk increases with the age at which ovarian endometriosis is diagnosed, and this increased risk is more significant in women aged ≥ 50 years.
Evidence also suggests that oophorectomy reduces ovarian cancer risk effectively. Investigators have generally reported that unilateral oophorectomy and radical extirpation of all visible endometriosis have a good protective effect on the subsequent development of ovarian cancer. Based on these data, active surgical intervention should be considered for older women suffering from endometriosis.
Although the general classification of the EOCs is often based on the morphology and pathologic features, such as serous, endometrioid, clear-cell, mucinous, transitional cell, and mixed types, it can also be divided into two subtypes according to their carcinogenesis: type I and type II.
Each type has a histological and molecular profile correlated with distinct clinical presentations, treatments, and survival outcomes. Type I tumors are slow growing and include low-grade serous carcinoma, mucinous carcinoma, endometrioid carcinoma, malignant Brenner tumor, and clear-cell carcinomas. Type II tumors are more aggressive and include high-grade serous carcinoma, malignant mixed mullerian tumors (carcinosarcomas), and undifferentiated carcinoma.
EAOCs represent a subclass of Type I ovarian neoplasms that primarily consist of endometrioid and clear-cell subtypes with endometriosis found in 30%-55% of clear-cell and 30%-40% of endometrioid ovarian cancers.
Furthermore, favorable factors of EAOC include early-stage disease, low-grade disease and a specific histology, showing less invasiveness and slow growth, which supports the epidemiologic evidence linking endometriosis to a precursor lesion of ovarian cancer. In spite of favorable characteristics of EAOC, there is no difference in prognosis between EAOC and non-EAOC when adjusted with stage and a specific histology which suggests that endometriosis may not affect the progression after the onset of ovarian cancer.
Treatment of endometriosis whether medical or surgical is selected based on the type and severity of the symptoms, as well as the patient’s age and her wish to preserve fertility. When analgesics, such as non-steroidal anti-inflammatory drugs are ineffective, oral contraceptives (OCs) or progestin may be used to relieve symptoms by suppressing the secretion of estrogen or acting directly on the lesion. Gonadotropin-releasing hormone agonists and antagonists may also be used when patients fail to respond to OCs or progestin. Thus, with the recent developments in therapies, surgery can often be avoided in many cases of endometriosis.
The management strategy of EAOCs and non-EAOCs seems to be similar, including a complete and thorough surgical intervention either by primary cytoreductive surgery or by interval cytoreductive surgery and followed by platinum-paclitaxel chemotherapy with/without anti-angiogenesis (bevacizumab) or target therapy (poly (ADP-ribose) polymerase inhibitors (iPARP)).
The improvement in ovarian cancer therapy achieved through intensive investigation of PARP inhibitors has boosted the research of new target therapies in ovarian cancer.
New approaches are likely to soon translate into clinical research. Since inflammatory and epigenetic processes seem to play a predominant role in the pathogenesis of endometriosis-associated ovarian carcinomas, immune checkpoint inhibitors and targeting the PI3K pathway as well as epigenetic treatment approaches may play an important role in the treatment of these tumors.
The identification of the molecular changes and mechanisms of EAOC will facilitate the development of more effective and tailored diagnostic modalities, leading to improved oncologic outcomes.
Conclusion
In spite of endometriosis being a benign disease, patients diagnosed with endometriosis may harbor an increased risk for developing ovarian cancer, that reasonably cannot be ignored and clinicians must be aware of that.
The application of molecular technology will provide us the opportunity to find out more about the etiology of endometriosis, and hopefully to identify biomarkers of EAOC risk in order to stratify the patients with endometriosis who are at risk of developing cancer, and to separate patients at risk from those who will continue to have benign disease.
While the investigation of these new technologies is in progress, clinicians should be aware of the increased risk of EAOC in high-risk populations and select appropriate preventive measures.
Meanwhile, we should inform and reassure our patients with endometriosis and as Vargas stated, “it is necessary to centralize patients with endometriosis and offer them the best possibilities for diagnosis and treatment”.















