Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.23 no.6 Lisboa dez. 2016
https://doi.org/10.1016/j.jpge.2016.03.001
CLINICAL CASE
Ampullary Metastasis From Breast Cancer: A Rare Cause of Obstructive Jaundice
Metástase Ampular de Neoplasia da Mama: Uma Causa Rara de Icterícia Obstrutiva
Sílvia Giestasa,*, Sandra Lopesa, Paulo Soutoa, Cláudia Agostinhoa, Ernestina Camachoa, Maria Ciprianob, Carlos Sofiaa
aGastroenterology Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
bPathology Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
* Corresponding author.
ABSTRACT
Breast cancer is the most common tumor in women and the first cause of death for malignancy in the female. Bile ducts are not among the common sites of metastasis from breast cancer. Few cases of obstructive jaundice due to metastatic breast cancer have been described in the literature and they mostly resulted from widespread liver metastases that eventually involved the bile ducts. We report an exceptional case of ampullary metastasis in the absence of liver metastases.
Sporadic reports have been published about the involvement of the ampulla by breast cancer metastasis. This case emphasizes the need to consider this diagnosis in women presenting with obstructive jaundice, especially when there is a clinical possibility of breast cancer.
Keywords: Breast Neoplasms; Ampulla of Vater; Jaundice, Obstructive/etiology; Neoplasm Metastasis
RESUMO
O cancro da mama é o tumor mais comum em mulheres e a principal causa de morte por neoplasia nesta população. A via biliar não é um local comum de metastização desta neoplasia. Poucos casos de icterícia obstrutiva devido a metástases mamárias têm sido descritos na literatura e ocorrem principalmente devido a metástases hepáticas que comprimem a via biliar. Relatamos um caso excepcional de metástase ampular na ausência de metástases hepáticas.
Existem apenas relatos esporádicos do envolvimento da ampola por metástase mamárias. Este caso enfatiza a necessidade de considerar este diagnóstico perante um quadro de icterícia obstrutiva, especialmente em doentes com possível neoplasia mamária.
Palavras-chave: Neoplasias da Mama; Ampola Hepatopancreática; Icterícia Obstrutiva/etiologia; Metástase Neoplásica
1. Introduction
Breast cancer is the most common malignancy in women, with over a million newly diagnosed cases each year and being one of the leading causes of cancer death among them.1–3
In 10% of the cases distant metastases are already present at the time of the diagnosis.4–6 Breast cancer metastases occur through contiguous, lymphatic and hematogenous spread. Common sites of metastasis include bone, lung, lymph nodes, liver and brain. Virtually every site of the human body can be targeted by hematogenous spread of breast cancer. However, metastases to the digestive tract, the kidneys and retroperitoneal organs have only been occasionally reported.4–6 Gastrointestinal tract involvement is detected in only 10% of all the cases.4–6 Widespread liver metastases that compress or infiltrate the bile ducts can sometimes cause obstructive jaundice, whilst a direct metastatic involvement of the extrahepatic bile ducts in absence of hepatic lesions is exceptional.4–6
We report a singular case of obstructive jaundice due to a metastatic breast cancer to the ampulla of Vater. To the best of our knowledge and review of the literature there have been only few similar reports.7–10
This case emphasize that diagnosis can be difficult and controversial when metastasis from breast cancer occurs at uncommon sites, but quick and accurate diagnosis is needed for an adequate treatment choice.
2. Case presentation
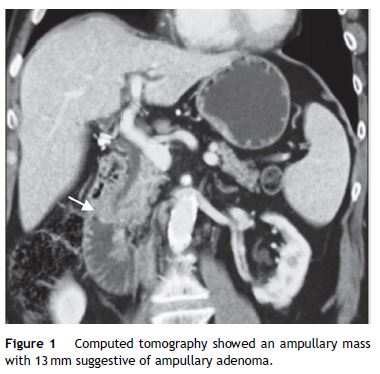
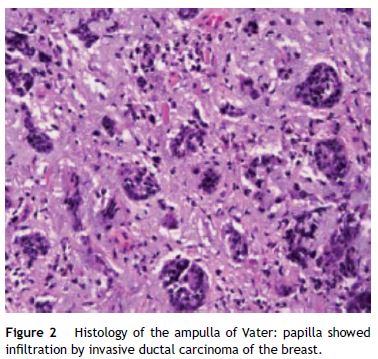
A 59-year-old female was admitted to the emergency department with mucocutaneous jaundice associated with pruritus. No dark urine, acholic stools, abdominal pain, fever, anorexia or weight loss were present. Personal history included systemic lupus erythematosus (treated with hydroxychloroquine sulfate and mycophenolate mofetil), type 2 diabetes mellitus non-insulin treated and a breast lump (detected about a month ago in breast cancer screening mammography) under investigation (histology ongoing). The laboratory tests showed an obstructive pattern with total bilirubin 9 mg/dL, direct bilirubin 6.4 mg/dL, aspartate aminotransferase 126 IU/L, alanine aminotransferase 208 IU/L, gamma glutamyl transpeptidase 796 IU/L, alkaline phosphatase 726 IU/L and negative inflammatory parameters. Blood tumor markers (cancer antigen 19.9 and carcinoembryonic antigen) were within normal ranges. The abdominal ultrasound study was limited by bowel gas interposition but allowed view marked dilatation of intrahepatic bile ducts and common bile duct 14 mm and could not define the cause of obstruction. Abdominal tomography confirmed dilatation of hepatic bile ducts (common bile duct with 15 mm without evidence of choledocholitiasis) and showed an ampullary mass with 13 mm of diameter suggestive of ampullary adenoma without suspicious abdominal lymph nodes (Fig. 1). Endoscopic retrograde cholangiopancreatography (ERCP) showed papilla of Vater with moderately increased volume and irregular mucosal suggestive of congestive ampullary adenoma. Biopsies were performed and two plastic biliary stents were placed. Histology showed infiltration by invasive ductal carcinoma of the breast (Fig. 2). During hospitalization the histology of the breast nodule revealed an invasive breast carcinoma and the patient initiated chemotherapy (including transtuzumab). Further evaluation with chest tomography and radionuclide bone scanning revealed the presence of bone metastases. For better palliation, three months after the plastic stent implantation, patient underwent repeat ERCP with the placement of a metal stent due to its better efficiency. At discharge and several weeks later the repeated laboratory tests revealed regression of cholestasis. The patient succumbed to metastatic disease 1 year later of the diagnostic without jaundice or abnormal liver function tests.
3. Discussion
Breast cancer is the most commonly diagnosed cancer worldwide, with more than 1,384,000 cases detected each year. It is the main cause of death from cancer in females and the second in the general population, after lung cancer.1–3
Distant metastases of breast carcinoma are present in about 10% of patients at the time of diagnosis, while 30% of patients will develop metastatic disease following surgery and/or chemotherapy, radiotherapy or endocrine therapy.3
The most common sites of breast cancer metastases are the bone, lung, liver and brain, while the involvement of gastrointestinal tract is rare and can pose a diagnostic challenge.4–6 Metastatic breast cancer can cause obstructive jaundice when multiple liver lesions are present, the bile duct is compressed by enlarged lymph nodes or, more rarely, when the head of the pancreas is involved.4–6 The biliary tract is very rarely affected by metastases in general, and in these cases, colorectal cancer is the most frequent malignancy involved. Other reported primary tumors causing metastatic biliary obstruction include malignant melanoma, lymphoma, gallbladder, stomach, esophagus, liver, ovary, cervix, uterus, muscle, kidney, prostate, bone and brain. Isolated breast cancer metastasis to the biliary tract, gallbladder and Vater ampulla are exceptional.4–6 It is important to recognize this group of patients because in patients with normal liver function, relief of biliary obstruction using surgical bypass or biliary stenting extends their survival to over 1 year, in comparison to those with liver metastases, whose mean survival is only about 1 month.11–13
By reviewing the literature there were only a few cases of extrahepatic biliary tract metastasis from breast cancer involving the periampullary area.7–10 A permanent feature of all the reports available in the literature is that the diagnosis was not achieved nor suspected before endoscopy biopsy and/or surgery. Similarly, in the majority of the cases there was a long interval between the diagnosis of the primary tumor and the development of metastases affecting the biliary tract. This timing can make it hard to suspect a relationship between the breast cancer and the biliary disease.7–10 In our case this was the first evidence of metastatic breast cancer. Patients with a history of malignancy found to have obstructive jaundice from an ampullary mass should undergo further investigation to determine primary biliary cancer versus metastatic disease in order to provide appropriate surgical and medical management. Nowadays, numerous modalities such as extracorporeal ultrasonography (US), esophagogastroduodenoscopy, biopsy, computed tomography (TC), magnetic resonance (MRI), endoscopic ultrasonography (EUS), ERCP, intraductal US and angiography are available for diagnosing ampullary neoplasms. Appropriate and efficient selection of such modalities is necessary for lessening procedure-related complications as well as the burden on the patients.14 It is not always possible to distinguish adenoma from carcinoma or metastatic involvement of the ampulla with duodenoscopy only, and histological evaluation by forceps biopsy is mandatory for establishment of a definitive diagnosis. The diagnostic accuracy of forceps biopsy in ampullary neoplasms reportedly ranges from 47 to 95%.14 Immunohistochemistry plays a key role for indicating the histological type of the tumor, since the metastases of breast carcinoma to the gastrointestinal tract have an endoscopic, radiological and histological aspect similar to the adenocarcinoma poorly differentiated with signet ring cells.10 Tumor stating with EUS and/or intraductal US can provide useful information for making therapeutic decisions, especially in the selection of patients for endoscopic papillectomy.14 CT/MRI is recommended for the detection of distant metastases. EUS can be performed in a single session with ERCP, biopsy and biliary stent placement (if indicated).14
Patients with metastatic breast cancer in the biliary tract need a long-term palliative treatment strategy. The use of novel chemotherapy, hormonal therapy and irradiation in conjunction with biliary decompression or surgical intervention may lead to even more prolonged survival and improve quality of life.15,16 A variety of chemotherapy strategies are available for metastatic breast cancer and should be tailored for each patient along with endocrine therapy in tumors positive for estrogen and progesterone receptors.16–18 HER2 receptor-positive metastatic disease should be treated with trastuzumab in addition to chemotherapy.19
Biliary stenting is a commonly used procedure in treating patients with pancreaticobiliary malignancies, metastatic disease and external biliary compression by lymph nodes. It is used both as a bridge to surgery in patients with resectable disease and for palliation in those with biliary obstruction caused by inoperable disease.20,21 For palliation endoscopic biliary drainage is effective in more than 80% of cases.21–25 Multiple trials have demonstrated that placement of self-expanding metallic stents in patients with malignant obstruction of the common bile duct offers higher technical and clinical success rates as well as lower complication rates and a superior cumulative stent patency when compared with plastic stent placement.21–25 Initial insertion of a plastic stent is most cost-effective if patient life expectancy is shorter than 4 months, if it is longer than 4 months then initial insertion of a self-expanding metallic stents is more cost-effective.21–25 In our case, we first placed a plastic stent to gain symptomatic relief, but after knowing the result of histology, we replaced it with a metal one 3 months after the first intervention.
The present report provides evidence that metastases from breast cancer can target the extrahepatic bile ducts in the absence of liver involvement, and the invasion of the bile duct wall can also be the first sign of advanced disease, thus making the diagnosis particularly difficult. Although gastrointestinal involvement in breast cancer is rare, in patients with a history of a breast lump, the possibility of biliary localization of metastatic disease should be always considered in the differential diagnosis of obstructive jaundice whose origin is unclear, especially because this condition is amenable to palliation and improve survival rate.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-917. [ Links ]
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [ Links ]
3. Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, et al. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst. 2011;103:1037-48. [ Links ]
4. Stellato TA, Zollinger RM, Shuck JM. Metastatic malignant biliary obstruction. Am Surg. 1987;53:385-8. [ Links ]
5. Franco D, Martin B, Smadja C, Szekely AM, Rougier P. Biliary metastases of breast carcinoma. The case for resection. Cancer. 1987;60:96-9. [ Links ]
6. Taal BG, den Hartog FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma. Gastrointest Endosc. 1992;38:136-41. [ Links ]
7. Titus AS, Baron AS, Todd H. Solitary breast metastasis to the ampulla and distal common. Am Surg. 1977;63:512-5. [ Links ]
8. Rego RF, Atiq M, Velchala N, Nevin D, McElreath DP, McKnight WD, et al. Ampullary metastasis from breast cancer: an unusual finding. Endoscopy. 2009;41:E278-9. [ Links ]
9. Ferrari AB, Pulcini G, Gheza F, Vinco A, Manenti S, Cervi E, et al. Duodenal metastasis from male breast cancer: a case report and review of the literature. J Med Case Rep. 2009;3:8331. [ Links ]
10. Bastos T, Souza TF, Otoch JP, Grecco E, Ávila F, Artifon ELA. Metastasis of breast cancer to major duodenal papilla. Rev Gastroenterol Peru. 2014;34:149-50. [ Links ]
11. Ellis M, Levey J. Endoscopic biliary drainage for breast carcinoma metastatic to the duodenum. Am J Gastroenterol. 2003;98:S167. [ Links ]
12. Budimir I, Pusic MS, Nikolic M, Dorosulic Z, Ljubicic N, Stajduhar E, et al. Obstructive jaundice as an uncommon manifestation of metastatic breast cancer. World J Oncol. 2015;6:297-300. [ Links ]
13. Pappo I, Feigin E, Uziely B, Amir G. Biliary and pancreatic metastases of breast carcinoma: is surgical palliation indicated. J Surg Oncol. 1991;46:211-4. [ Links ]
14. Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J. Diagnosis of ampullary cancer. Dig Surg. 2010;27:115-8. [ Links ]
15. Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. [ Links ]
16. Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol. 2014;25:1871-88. [ Links ]
17. Carrick S, Parker S, Thornton CE, Ghersi D, Simes J, Wilcken N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009;2:CD003372. [ Links ]
18. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431-42. [ Links ]
19. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. [ Links ]
20. Chun HJ, Kim ES, Hyun JJ, Kwon YD, Keum B, Kim CD. Gastrointestinal and biliary stents. J Gastroenterol Hepatol. 2010;25:234-43. [ Links ]
21. Perdue DG, Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J Clin Gastroenterol. 2008;42:1040-6. [ Links ]
22. Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213-21. [ Links ]
23. Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458-73. [ Links ]
24. Cochrane J, Schlepp G. Metastatic breast cancer to the common bile duct presenting as obstructive jaundice. Case Rep Gastroenterol. 2015;9:278-84. [ Links ]
25. Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article
Conflicts of interest
The authors have no conflicts of interest to declare.
* Corresponding author.
E-mail address: silviagiestas@gmail.com (S. Giestas).
Received 13 November 2015; accepted 8 March 2016














