Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
GE-Portuguese Journal of Gastroenterology
versión impresa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.2 Lisboa abr. 2018
https://doi.org/10.1159/000479432
CLINICAL CASE STUDY
Combined Extensive Esophageal Squamous Papillomas and Florid Cardiac Gland Hyperplasia in a Patient with Adenocarcinoma
Extensos papilomas escamosos esofágicos combinados com uma florida hiperplasia glandular do cárdia num doente com adenocarcinoma
Takanori Suganumaa, Han-Seung Yoonb
aIida Municipal Hospital, Iida, and bNagasaki University Graduate School of Biomedical Science, Sakamoto, Nagasaki, Japan
* Corresponding author.
ABSTRACT
We report a rare case of extensive esophageal squamous papillomas (ESPs) involving the entire esophagus and florid cardiac gland hyperplasia involving only the lower esophagus in a 39-year-old woman with heartburn and epigastric distress for the past 2 years. Previous esophagogastroduodenoscopy showed multiple ESPs involving the entire esophagus extending 38 cm from the esophageal orifice to the esophagogastric junction (EGJ). Additionally, prominent cardiac gland hyperplasia over the esophageal posterior wall was exhibited extending 12 cm from the mid-esophagus to the EGJ. A biopsy obtained from the ESP area showed typical squamous papillomas and cardiac gland hyperplasia with no evidence of koilocytosis or malignancy. Polymerase chain reaction was negative for a variety of human papilloma virus DNAs. Subsequently, Siewert type II gastric cancer with submucosal elevation of the stomach was detected at the EGJ. Endoscopy showed a 20-mm-thick lesion appearing to extend to the muscularis propria; subsequent biopsy showed invasive adenocarcinoma. Total gastrectomy with D2 lymph node dissection, splenectomy, and Roux-en-Y reconstruction were performed for the EGJ cancer. The patient died from widespread multiorgan metastasis within 2 years following surgery.
Keywords: Esophagus,·Squamous papilloma, Cardiac gland hyperplasia,· Esophagogastric junction adenocarcinoma
RESUMO
Descrevemos um caso raro de extensos papilomas escamosos esofágicos (PEEs) envolvendo todo o esófago associados a uma florida hiperplasia glandular do cárdia envolvendo apenas o esófago distal, numa mulher de 39 anos com quadro de 2 anos de pirose e dispepsia. Endoscopias altas prévias mostraram múltiplos PEEs envolvendo todo o esófago desde o cricofaríngeo até à junção esofagogástrica (JEG). Adicionalmente, verificou-se também uma proeminente hiperplasia glandular do cárdia na parede posterior esofágica com uma extensão de cerca de 12 cm, desde o esófago médio até à JEG. Biopsias destas áreas evidenciaram os típicos PEEs e hiperplasia glandular do cárdia sem evidência de coilocitose ou malignidade. A análise por polymerase chain reaction foi negativa para o DNA de vários vírus do papiloma humano. Subsequentemente foi diagnosticado um cancro juncional tipo Siewert II com elevação da submucosa a nível da vertente gástrica da junção. A endoscopia mostrou uma lesão de 20 mm de espessura que parecia já estender-se para a muscular própria; biopsias confirmaram o diagnóstico de adenocarcinoma invasor. Foi realizada gastrectomia total com dissecção ganglionar D2, esplenectomia e reconstrução em Y de Roux para cancro da junção esofagogástrica. O doente morreu dois anos após a cirurgia com metástases multi-orgânicas.
Palavras-Chave: Esófago, Papiloma escamoso, Hiperplasia glandular do cárdia, Adenocarcinoma da junção esofagogástrica
Introduction
Although esophageal squamous papilloma (ESP) is a rare disease, improvements in endoscopy technique have led to higher rates of detection. The prevalence of ESP is reported to be 0.2% in the general Japanese population [1] and 4.5% in cases of benign esophageal lesions [2]. ESP is classified as a benign squamous epithelial polypoid tumor, and typically identified as a solitary lesion in the lower esophagus. The pathogenesis and biological characteristics of ESPs are not clear. There are few reports of cases with frequent ESP occurrence and only 2.6% of cases exhibit multiple ESPs [3].
Aside from this, the structure of the cardiac mucosa (epithelium) at the esophagogastric junction (EGJ) has been subject to controversy. Some view cardiac mucosa as a normal structure present at birth [4], whereas others suggest that it develops as a metaplastic response to gastroesophageal reflux disease [5]. Regardless, when cardiac mucosa is present, it usually extends a few millimeters below and above the Z line [6]. Shimoda and colleagues [7, 8] reported the presence of cardiac mucosa at the proximal area below the EGJ measuring 12–36 mm in length (average: 10.4 mm) in all 131 esophagectomy specimens and at the distal area above the EGJ measuring 0.3–22 mm (average: 4.6 mm) in length in 125 specimens (95.4%). Most recent reports agree with the notion that cardiac mucosa is present over the EGJ, in both the proximal stomach and distal esophagus. The former is denoted as gastric cardiac mucosa, whereas the latter is esophageal.
Here, we present a rare case of extensive ESPs involving the entire esophagus and prominent cardiac gland hyperplasia involving the lower esophagus, which were subsequently complicated by adenocarcinoma of the EGJ [9, 10].
Clinical Case
A 39-year-old woman had a 2-year history of heartburn and epigastric distress. The patient regularly took lansoprazole and rebamipide and used oxethazaine for stomachache. Previous esophagogastroduodenoscopy performed in June 2012 had indicated multiple ESPs (Fig. 1a–d). Follow-up ESP screening by endoscopy was performed in April 2014. Follow-up endoscopy identified numerous ESPs over the posterior wall of most of the esophagus, extending 38 cm from the laryngeal arytenoid region and esophageal orifice to the EGJ. Interestingly, in a 12-cm portion from the mid-esophagus to the EGJ, the background mucosa of the distal esophagus was hyperemic and distinctly different from squamous epithelium or ESPs. The mucosa appeared very similar to the cardiac type of gastric mucosa (defined as cardiac mucosa here) and was mixed with multiple ESPs extending 12 cm from the mid-esophagus (26 cm from incisors) to the EGJ (Fig. 2a, b).
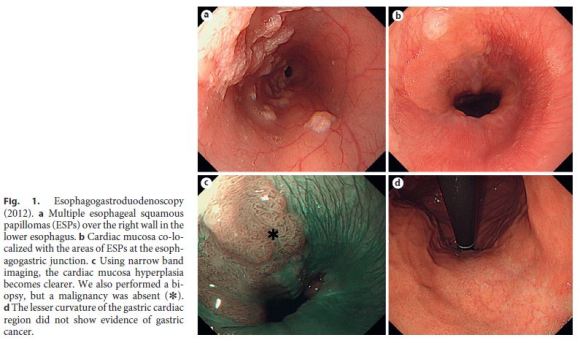

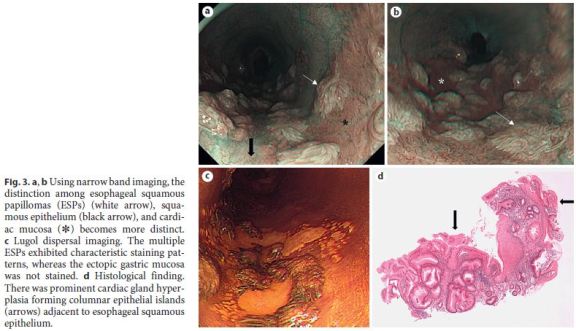
Using narrow band imaging, the distinction between the ESP, squamous epithelium, and cardiac mucosa became clearer (Fig. 3a, b). Using Lugol dispersal imaging, the ESPs exhibited characteristic staining patterns, whereas cardiac mucosa was not stained (Fig. 3c). Biopsy from this area showed both squamous papillomas and cardiac mucosa with prominent glandular hyperplasia. The cardiac glandular hypertrophy was sufficiently florid to directly expose the inner lumen of the esophagus, forming columnar epithelial islands (Fig. 3d, arrows). There was no evidence of koilocytosis or dysplasia/malignancy in the squamous papillomas. Neither active inflammation nor intestinal metaplasia was seen. The results of polymerase chain reaction (PCR) for the DNA of various types of human papilloma virus (HPV types 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 56, 58, and 59) were negative. Neither immunohistochemistry using p16 nor in situ hybridization for HPV DNA yielded positive results.
On endoscopy examination, the lesser curvature of the gastric cardiac region showed an area of hemorrhagic mucosa, approximately 20 mm in size, associated with submucosal elevation. The hemorrhagic lesion was thickened, suggesting invasion to the submucosa (Fig. 2c). There was a significant, trench-like depression immediately beneath the EGJ, suggesting an invasion of approximately 1 cm into the esophagus. In addition, there was a papilloma in the part of the oral side of the lesion. Upper gastrointestinal endoscopy revealed minimal change esophagitis and a small hiatal hernia (Fig. 2d). Biopsy from the hemorrhagic gastric mucosa revealed adenocarcinoma (Tub2+Tub1), with focal areas showing invasion to the muscularis mucosae. Based on these findings, we diagnosed Siewert type II gastric cancer with submucosal elevation detected at the EGJ. Neither gastric biopsy nor serum antibodies were positive for Helicobacter pylori. Computed tomography did not reveal lymph node metastasis. Therefore, total gastrectomy with D2 lymph node dissection, splenectomy, and Roux-en-Y reconstruction was performed to resect the EGJ cancer.
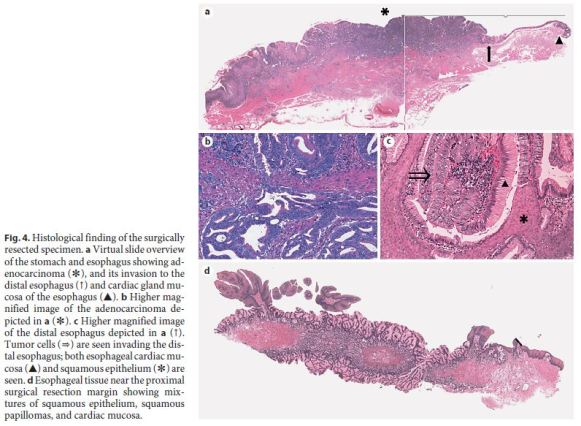
Using the Japanese Classification of Gastric Carcinoma (3rd English edition) [11], the malignant lesion of the EGJ (GE Siewert type II, less, type 1, 32 × 40 mm) was classified as an adenocarcinoma, stage IIIB ([Tub2>Tub1>Por1+Por2], pT3 [SS], int, INFc, ly3, v1, pN3a, pPM0, and pDM0). According to the TNM classification, the tumor was stage IIIC (Fig. 4a, b). The cancer measured 40 mm in length along the lesser curvature with a central tumor portion being 18 mm distal to the EGJ and showed invasion into the distal esophageal mucosa, up to 2 mm from the EGJ where squamous epithelium and hyperplastic cardiac mucosa were seen. Figure 4c shows cancer cells replacing cardiac mucosal glands adjacent to squamous epithelium. In addition, the distal esophagus showed mixtures of squamous epithelium, squamous papillomas, and cardiac mucosa (Fig. 4d). The cardiac mucosa was not completely circumferential around the distal esophagus, but showed skip distribution forming columnar epithelial islands, polypoid structures, or subepithelial islands. The cardiac mucosa consisted largely of mucous cells that were strongly positive for PAS and of very occasional parietal cells. Esophageal cardiac mucosa measured at least 18 mm in length above the EGJ. The proximal esophageal surgical margins were free from carcinoma cells, but contained mixtures of squamous papillomas, squamous epithelium, and hyperplastic cardiac mucosa (Fig. 4d).
Immunohistochemistry showed that esophageal cardiac glands were positive for MUC-5AC and MUC-6 and negative for MUC-1, MUC-2, CDX-2, and p53 (data not shown). Carcinoma cells were strongly positive for p53 and focally positive for MUC-5AC and MUC-6. Tumor cells were completely negative for MUC-1, MUC- 2, and CDX-2. The immunohistochemical profiles of the cancer cells appear to be similar to those of the hyperplastic esophageal cardiac glands.
Despite aggressive postoperative chemotherapy, the patient died from widespread multiorgan metastasis within 2 years following surgery.
Discussion
This is a rare report of extensive esophageal papillomatosis and prominent esophageal cardiac gland hyperplasia associated with cancer of the EGJ [16, 17].
The first report of ESP, with histological confirmation, was provided by Adler et al. [18] in 1959. ESPs are classified into three histological types: exophytic, endophytic, and spiked. In our case, ESPs were the exophytic type [14]. The critical characteristic of our case is the occurrence of multiple ESPs in the same areas of the esophagus as cardiac gland hyperplasia, with later development of adenocarcinoma of the EGJ.
Chronic esophageal inflammation secondary to gastroesophageal reflux disease or hiatal hernia have been suggested as etiological factors for both ESP [15] and cardiac gland hyperplasia [6]. In the present case, although endoscopy did not detect severe reflux esophagitis, an esophageal hiatal hernia was present and the patient had experienced heartburn and epigastric distress for 2 years. Furthermore, a deglutition reflex was clearly detected by endoscopy. Thus, the present case is considered to be associated with esophagitis. Histological biopsy and surgical esophageal specimens showed a mild form of chronic esophagitis; however, active severe esophagitis was not seen.
Cardiac gland hyperplasia originates from chronic stimulation of the cardiac gland, which is localized at the esophageal opening. Chronic esophageal inflammation results in hyperplasia of the lacunar epithelium in the crypt of the esophageal cardiac gland, which becomes exposed to the outer layer of the esophageal mucosa [7, 19– 21]. The process of hyperplasia extends from the lower esophagus to its central portion, which becomes the beginning of Barretts epithelium. In the present case, hyperplasia appeared to have occurred in the nearby mucosal tissues. Cardiac gland hyperplasia was only identifiable on the previous endoscopy at the EGJ (Fig. 1a–c); thus, it appeared to have spread from the lower esophagus following the development of ESPs.
The extent with which HPV infection contributes as an etiological factor for ESPs remains controversial. The prevalence of HPV-positive ESP was 10.5% in a report by Takeshita et al. [1]; the HPV genotypes 6 and 11 are the major subtypes in ESPs [12]. Koilocytosis, which is a histological marker indicative of viral infection, can occasionally be detected in cases where HPV infection is not detected by PCR. This might indicate that koilocytosis may not always be related to viral infection and that the possibility of infection by other unknown subtypes of the HPV virus cannot be excluded [12, 13]. In our case, while HPV infection was not detected by either the PCR or p16 immunohistochemistry methods, the possibility of HPV infection cannot be completely ruled out as the detection rate of HPV by DNA analysis remains low [22]. The relationship between EPS and the adenocarcinoma of the EGJ in our case is not clear. However, since the immunohistochemical profiles of the cancer and nonneoplastic cardiac glands were similar, except for the high expression of p53 in the cancer cells, nonneoplastic cardiac glands could provide a precursor lesion for cancer development later. Thus, based on this case, we inferred that cardiac hyperplasia is also a precursor of gastric adenocarcinoma.
In summary, the present case is a report of very rare esophageal papillomatosis and hyperplastic cardiac mucosa associated with adenocarcinoma of the EGJ.
References
1 Takeshita K, Murata S, Mitsufuji S, Wakabayashi N, Kataoka K, Tsuchihashi Y, et al: Clinicopathological characteristics of esophageal squamous papillomas in Japanese patients – with comparison of findings from Western countries. Acta Histochem Cytochem 2006;39:23–30. [ Links ]
2 Terada T: A clinicopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol 2013;2:191–198. [ Links ]
3 Winkler B, Capo V, Reumann W, Ma A, La Porta R, Reilly S, et al: Human papillomavirus infection of the esophagus. A clinicopathologic study with demonstration of papillomavirus antigen by the immunoperoxidase technique. Cancer 1985;55:149–155. [ Links ]
4 De Hertogh G, Van Eyken P, Ectors N, Geboes K: On the origin of cardiac mucosa: a histological and immunohistochemical study of cytokeratin expression patterns in the developing esophagogastric junction region and stomach. World J Gastroenterol 2005;11:4490–4496. [ Links ]
5 Chandrasoma P: Controversies of the cardiac mucosa and Barretts oesophagus. Histopathology 2005;46:361–373. [ Links ]
6 Fenoglio-Preister CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG: Gastrointestinal Pathology: An Atlas and Text, ed 3. Philadelphia, Wolters Kluwer/Lippincott Williams and Wilkins, 2007, pp 11–12. [ Links ]
7 Nakanishi Y, Saka M, Eguchi T, Sekine S, Taniguchi H, Shimoda T: Distribution and significance of the oesophageal and gastric cardiac mucosae: a study of 131 operation specimens. Histopathology 2007;51:515–519. [ Links ]
8 Shimoda T: Recent pathological knowledge of esophago-gastric junction, Barretts esophagus and its carcinoma. J Jpn Gastroenterol Soc 2008;105:11–26. [ Links ]
9 Attila T, Fu A, Gopinath N, Streutker CJ, Marcon NE: Esophageal papillomatosis complicated by squamous cell carcinoma. Can J Gastroenterol 2009;23:415–419. [ Links ]
10 Reed PA, Limauro DL, Brodmerkel GJ Jr, Agrawal RM: Esophageal squamous papilloma associated with adenocarcinoma. Gastrointest Endosc 1995;41:249–251. [ Links ]
11 Japanese Gastric Cancer Association: Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101– 112. [ Links ]
12 Orlowska J, Jarosz D, Gugulski A, Pachlewski J, Butruk E: Squamous cell papillomas of the esophagus: report of 20 cases and literature review. Am J Gastroenterol 1994;89:434–437. [ Links ]
13 Carr NJ, Monihan JM, Sobin LH: Squamous cell papilloma of the esophagus: a clinicopathologic and follow-up study of 25 cases. Am J Gastroenterol 1994;89:245–248. [ Links ]
14 Odze R, Antonioli D, Shocket D, Noble- Topham S, Goldman H, Upton M: Esophageal squamous papillomas. A clinicopathologic study of 38 lesions and analysis for human papillomavirus by the polymerase chain reaction. Am J Surg Pathol 1993;17:803–812. [ Links ]
15 Mosca S, Manes G, Monaco R, Bellomo PF, Bottino V, Balzano A: Squamous papilloma of the esophagus: long-term follow up. J Gastroenterol Hepatol 2001;16:857–861. [ Links ]
16 Lavergne D, de Villiers EM: Papillomavirus in esophageal papillomas and carcinomas. Int J Cancer 1999;80:681–684. [ Links ]
17 zur Hausen H: Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342–350. [ Links ]
18 Adler RH, Carberry DM, Ross CA: Papilloma of the esophagus. Association with hiatal hernia. J Thorac Surg 1959;37:625–635. [ Links ]
19 Tajima Y, Nakanishi Y, Yoshino T, Shimoda T: Clinicopathological study of early adenocarcinoma of the gastric cardia: comparison with early adenocarcinoma of the distal stomach and esophagus. Oncology 2001;61:1–9. [ Links ]
20 Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK: The histologic spectrum of Barretts esophagus. N Engl J Med 1976;295:476– 480. [ Links ]
21 Herlihy KJ, Orlando RC, Bryson JC, Bozymski EM, Carney CN, Powell DW: Barretts esophagus: clinical, endoscopic, histologic, manometric, and electrical potential difference characteristics. Gastroenterology 1984;86:436–443. [ Links ]
22 Syrjänen K, Syrjänen S: Detection of human papillomavirus in esophageal papillomas: systematic review and meta-analysis. APMIS 2013;121:363–374. [ Links ]
Statement of Ethics
This case complies with the ethical standards of the human experimentation committee responsible and is in accordance with the World Medical Association and the Declaration of Helsinki. The authors followed the protocols of their institutions to access the patient data. The family of the patient authorized the submission and publication of this work.
Disclosure Statement
The authors declare that they have no conflicts of interest.
* Corresponding author.
Dr. Takanori Suganuma
Iida Municipal Hospital
438 Yawatamachi
Iida, Nagano 395-8502 (Japan)
E-Mail takanorisuganuma@nifty.com
Received: March 31, 2017; Accepted after revision: May 26, 2017














