Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.2 Lisboa Apr. 2019
https://doi.org/10.1159/000488505
ORIGINAL ARTICLE
Effect of Meal Ingestion on Liver Stiffness and Controlled Attenuation Parameter
Efeito da ingestão de uma refeição sobre a elastografia hepática transitória e o parâmetro de atenuação controlado
Marco Silva, Pedro Costa Moreira, Armando Peixoto, Ana Luísa Santos, Susana Lopes, Regina Gonçalves, Pedro Pereira, Hélder Cardoso, Guilherme Macedo
Department of Gastroenterology, Centro Hospitalar de São João, Porto Medical School, Porto, Portugal
* Corresponding author.
ABSTRACT
Background and Aims: Despite the increasing use of noninvasive methods for the assessment of liver fibrosis and steatosis, the effect of fasting and food intake on these parameters is not yet clear. Our aims were to evaluate the effect of food intake on liver stiffness (LS) (measured by transient elastography) and controlled attenuation parameter (CAP) in patients with different degrees of liver disease and healthyvolunteers, and secondarily, to assess possible factors associated with variations of LS and CAP. Methods: We performed a prospective single-center study including patients with liver disease and healthy volunteers. LS and CAP were evaluated using FibroScan® (Echosens, Paris, France), before (fasting ≥8 h) and 30 min after intake of a standardized breakfast. We used common cutoffs for LS: > 7 kPa for significant fibrosis (F2 to F4) and > 11 to 14 kPa (mean 12.5 kPa) for cirrhosis. Results: Fifty-nine (72%) patients with liver disease and 22 (28%) healthy volunteers were included. LS significantly increased 30 min after food intake (pre-meal 6.1 kPa [IQR: 4.7–9.8] vs. after-meal 6.8 kPa [IQR: 5.5–10.6]; p < 0.001). This difference was only significant in patients with chronic liver disease (p = 0.02) and not in healthy volunteers (p = 0.106). CAP values did not increase significantly after food intake. Gender, body mass index, mass of body fat, lean body mass, and percent of body fat were not related with significant variations of LS and CAP values after meal intake. Conclusions: Significant variations of LS were observed after ingestion of a standard meal, which may have consequences for patient management. CAP values were not significantly affected by food intake. Therefore, we consider that before the isolated evaluation of CAP, it is not necessary to perform any fasting period.
Keywords: Liver disease, Healthy volunteers, Fibroscan
RESUMO
Introdução e Objetivos: Apesar do uso crescente de métodos não invasivos para a avaliação da fibrose hepática e da esteatose, ainda não está claro o efeito do jejum e da ingestão alimentar na determinação destes parâmetros. Os nossos objetivos foram avaliar o efeito da ingestão de alimentos sobre a rigidez hepática (LS) (medida por elastografia transitória) e o parâmetro de atenuação controlada (CAP) em doentes com diferentes graus de doença hepática e voluntários saudáveis e, secundariamente, avaliar possíveis fatores associados às variações de LS e CAP. Métodos: Realizámos um estudo prospetivo, num único centro, incluindo doentes com doença hepática crónica e voluntários saudáveis. A LS e o CAP foram determinados com a utilização de FibroScan®, antes (jejum ≥8 h) e 30 minutos após a ingestão de um pequeno-almoço padronizado. Foram usados pontos de corte comuns para a elastografia: > 7 kPa para fibrose significativa (F2 a F4) e > 11–14 kPa (média 12.5 kPa) para cirrose. Resultados: Cinquenta e nove (72%) doentes com doença hepática crónica e 22 (28%) voluntários saudáveis foram incluídos. A LS aumentou significativamente 30 minutos após a ingestão de alimentos (pré-refeição 6.1 kPa [IQR: 4.7–9.8] versus após a refeição 6.8 kPa [IQR: 5.5–10.6], p < 0.001). Essa diferença foi significativa em doentes com doença hepática crónica (p = 0.02) mas não nos voluntários saudáveis (p = 0.106). Os valores da CAP não variaram significativamente após a ingestão de alimentos. O género, o IMC, a massa de gordura corporal, a massa corporal magra e a percentagem de gordura corporal não se associaram a variações significativas dos valores de LS e CAP após a ingestão da refeição. Conclusões: Verificaram-se variações significativas da LS após a ingestão de uma refeição padrão, o que pode ter consequências na avaliação dos doentes. Os valores da CAP não foram significativamente afetados pela ingestão de alimentos. Por isso, consideramos que perante a avaliação isolada de CAP não é necessário a realização de qualquer período de jejum.
Palavras-Chave: Doença hepática, Voluntários saudáveis, Fibroscan
Introduction
The concept of staging had been borrowed from the world of clinical oncology, where the stages give an idea of how far it has spread through the body. This information is both a powerful indicator of prognosis as well as an important determinant of the most appropriate therapy. In liver disease, the stage is a measure of the extent of fibrosis and architectural disruption indicating how far the hepatitis has come along a hypothetical path from normal liver to cirrhosis. Although the gold standard for fibrosis staging is liver biopsy [1], noninvasive methods have been developed over the recent years for the evaluation of fibrosis, such as Fibroscan® (Echosens, Paris, France) [2]. Steatosis is the accumulation of lipid droplets within hepatocytes and is considered pathologic when it affects more than 5% of hepatocytes.
Steatosis initially exhibits an acinar zone 3 (centrilobular) distribution in adults [3]. It can act as a cofactor of fibrogenesis in patients with chronic liver disease of various etiologies such as chronic hepatitis C and B virus infection and alcoholic liver disease [4, 5]. Steatosis can also be diagnosed by noninvasive means, mainly using conventional imaging techniques, like computed tomography scan, magnetic resonance imaging, and ultrasonography [6]. However, these are costly and some are not easily available, not standardized, or have controversial performance [6]. Recently, the controlled attenuation parameter (CAP) was developed and demonstrated good accuracy for the detection and quantification of steatosis in patients with chronic liver disease from various etiologies [7, 8]. Furthermore, CAP is measured simultaneously with liver stiffness (LS) using transient elastography (TE) [2, 9]. However, it is difficult for clinicians to interpret CAP results, since validity criteria are not clearly defined [9]. A recent study by Andrade et al. [10] including 159 liver biopsies showed that optimal CAP cutoff values for detecting steatosis ≥S1, ≥S2, and ≥S3 were 206.5, 232.5, and 282.5 dB/m, respectively.
Results of LS are expressed in kPa and can range from 2.5 to 75 kPa [11]. Cutoff values for diagnosing significant fibrosis (≥F2) or cirrhosis (F4) vary depending on the underlying liver disease. However, commonly used cutoffs in clinical settings are >7 kPa for significant fibrosis (F2 to F4) and >11 to 14 kPa (mean 12.5 kPa) for cirrhosis [12–14].
Biliary obstruction, congested liver, and high alanine aminotransferase and bilirubin levels are well-known factors that may induce false-positive results in LS assessment using TE but, the accuracy of CAP to detect moderate to severe steatosis seems not to be influenced by these factors [6]. On the other hand, high body mass index (BMI), alcohol drinking, F3–4 fibrosis, and histological steatosis grade 3 interfere with the accuracy of CAP measurement [9, 15]. Also, previous studies demonstrated that LS assessed by TE was affected by food ingestion from the mechanism of postprandial hyperemia [16–18]. Another study demonstrated a significant alteration in LS only in a subgroup of patients with chronic HBV infection without significant fibrosis, and the authors concluded that the absence of fasting would not imply a change in the clinical decision [19]. Similarly, the effect of food intake on CAP is not yet clear. In the study by Ratchatasettakul et al. [20], the CAP values were significantly reduced after a liquid meal intake (15–120 min), returning to the fasting levels 150 min later.
As such, we aimed to evaluate the effect of food intake on LS (measured by TE) and CAP. Secondarily, we evaluated possible anthropometric, clinical, and biological data associated with these possible effects.
Material and Methods
We performed a single-center prospective study between March and November 2016, including patients with different stages of liver disease who came to our gastroenterology department for TE and healthy volunteers (apparently healthy employees or students of our hospital without known liver disease). We excluded from the study individuals in whom the fibroscan was not able to obtain results with accuracy (e.g., those having congestive heart failure, pregnancy, ascites, hepatitis, and/or cholestasis jaundice [aspartate aminotransferase or alanine aminotransferase >five times the upper normal, total bilirubin >5 mg/dL]) [20].
The study was conducted according to the principles of the Declaration of Helsinki. The study was reviewed and approved by the local ethics committee “Comissão de Ética para a Saúde do Centro Hospitalar São João/Faculdade de Medicina da Universidade do Porto.”
LS and CAP were evaluated using FibroScan® (Echosens, Paris, France) before (fasting ≥8 h) and 30 min after intake of a standardized breakfast (milk, coffee, sugar, bread, butter, cookies – with ∼600 kcal). Although the use of only 2–4 h of fasting was described [16, 17, 21], in our study, an overnight fasting period (nearly 8 h) was used in order to ensure that there was definitively no bias with food ingestion prior to the evaluation, as described in other articles [18, 20, 22, 23].
Anthropometric and body composition measurement (Tanita® TBF-300A, Arlington Heights, IL, USA) including BMI, mass of body fat, lean body mass, and percent of body fat were obtained on the same day of LS and CAP assessment.
Two experienced (over 300 cases) operators performed all LS and CAP measurements, using standard M probe, and following international recommendations and those of the provider of the instrumentation [24]. TE is performed on a patient in supine position, with the right arm elevated to facilitate access to the right liver lobe. The tip of the probe is contacted to the intercostal skin with coupling gel in the 9th to 11th intercostal space, considering as valid measurements 10 successful acquisitions with a success rate >60%, and with an interquartile range (IQR) < 30% [25].
CAP was evaluated using the same radiofrequency data and region of interest used for the assessment of LS. LS and CAP values were expressed in kilopascal (kPa) and dB/m, respectively. CAP was evaluated simultaneously with LS determination, and the CAP values were considered only when the LS values were valid.
For descriptive analysis, categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and IQRs for variables with skewed distributions. All reported p values are two-tailed, with a p value of < 0.05 indicating statistical significance. Analyses were performed with the use of SPSS software version 22.0.
Results
During the period of the study, 85 cases were included. Four (5%) patients with inconclusive LS determination were excluded.
Baseline characteristics and biochemical data are shown in Table 1. The majority of patients were male (69%). The mean age was 51 ± 13 years. The mean BMI was 26.1 ± 4.2, and the mean percentage mass of body fat was 23.4 ± 9.5%.
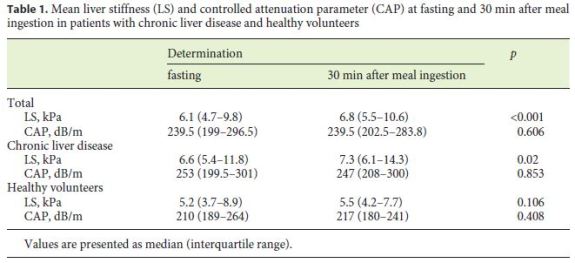
Overall, 59 (73%) patients with chronic liver disease – among whom 13 (22%) with cirrhosis – and 22 (27%) healthy volunteers were included. The etiologies of liver disease were NAFLD/NASH (34%), alcohol (31%), HCV (25%), and HBV infection (10%).
LS significantly increased 30 min after food intake (6.1 kPa [IQR: 4.7–9.8] vs. 6.8 kPa [IQR: 5.5–10.6]; p < 0.001). This difference was only significant in patients with chronic liver disease (p = 0.02) and not in healthy volunteers (p = 0.106) (Table 1). An intraindividual analysis showed that 4 (17%) patients with initial LS of 6–10 kPa and 11 (28%) patients with initial LS < 6 kPa had an aftermeal LS of > 10 and > 6 kPa, respectively. On the other hand, 2 (11%) patients with initial LS > 10 kPa and 2 (9%) patients with initial LS of 6–10 kPa had an after-meal LS of < 10 and < 6 kPa, respectively.
CAP values did not vary significantly after food intake. However, 8 (25%) patients with initial CAP < 222 dB/m had an after-meal CAP ≥222 dB/m. On the contrary, 5 (11%) patients with initial CAP ≥222 dB/m had an aftermeal CAP < 222 dB/m.
Gender, etiology of liver disease, BMI, lean body mass, and fat body mass were not related with significant changes on LS and CAP values after meal intake.
Discussion
In the last decade, TE became a valuable tool for the noninvasive assessment of fibrosis in chronic liver diseases, with obvious advantages in the allocation of patients on different classes of disease progression [25, 26]. While advanced fibrosis and cirrhosis are detected with high sensitivity and specificity, the discrimination of early stages of fibrosis by TE remains difficult [24].
TE is increasingly becoming a routine procedure in clinical practice in several countries and is already considered in treatment guidelines. However, small differences in TE values (such as 2–3 kPa) may have an impact in identifying cases of significant fibrosis, reinforcing the need for quality standardization [27].
The increase in LS after a meal is possibly a consequence of increased liver blood flow after food intake. Previous studies showed this effect by measure of liver blood flow using Doppler ultrasound [28]. Also, Dauzat M et al. [29] analyzed the volume of blood flow in portal vein after the intake of a fluid meal and showed that the blood flow already increased at 15 min and reached a maximum 30 min after food intake. Berzigotti et al. [18] measured LS, portal vein vascular flow, and hepatic artery vascular flow after meal intake in cirrhotic patients. Postprandial hyperemia was accompanied by a marked increase in LS and these differences directly correlated with hepatic artery vascular flow (but surprisingly were not associated with differences in portal vein vascular flow).
In our study, we found a significant statistical difference in TE values in patients with chronic liver disease (p = 0.02). These data are in accordance with previous works [16–18]. In fact, in the study by Arena et al. [17], with a fibrosis stage validated by histology, a higher LS value was found between 15 and 45 min after meal intake in all patients, with more pronounced differences in cirrhotic individuals.
We verified that 11 patients (28%) with ET 6 kPa 30 min after the standardized meal. LS values of 6–7 kPa to be sufficient for the diagnosis of significant fibrosis [31]. Such differences in patient stratification may lead to unnecessary treatments (taking into account the presumption of significant fibrosis).
In the study by Ratchatasettakul et al. [20], a significant decline of CAP values following meal intake was observed at 15–120 min. We have not found the same effect of meal intake on CAP values. In our study, the timing of the measurements was set at 30 min after meal ingestion, while in the study by Ratchatasettakul et al. [20], the CAP postmeal peak was observed 60 min after meal intake. Our time point was chosen according to previous observations indicating that postprandial hyperemia and the postprandial increase of portal pressure were highest 30 min after meal ingestion [18]. Also, contrary to this study and previous studies that have used a liquid meal with 600 kcal as a standardized measure [28, 29], in our study, we preferred the adoption of a solid meal with equal caloric load, in order to bring the study conditions closer to realworld practice. Meal composition may also have played some role in LS and CAP determinations, and thus, it may explain some differences between these studies. However, further studies are necessary to determine the real role of meal composition in the assessment of CAP and LS values.
The lack of later time points is a limitation of our study that makes it difficult to specify how long the fasting period before CAP and LS assessment should be. Our data did not demonstrate a statistically significant difference between the values of CAP obtained in the fasting period and after a standard meal. Therefore, we consider that before the isolated evaluation of CAP, it is not necessary to perform any fasting period.
This type of prospective study could have a central impact in the adoption of better resource management and scheduling strategies in diagnostic centers.
References
1 Guido M: Chronic hepatitis: grading and staging; in Saxena R (ed): Practical Hepatic Pathology – A Diagnostic Approach, Saunders, 2011, p 201. [ Links ]
2 Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, de Ledinghen V, Trinchet JC, Beaugrand M: Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat 2012;19:244–253. [ Links ]
3 Yeh MM, Brunt EM: Pathological features of fatty liver disease. Gastroenterology 2014;147:754–764. [ Links ]
4 Powell EE, Jonsson JR, Clouston AD: Steatosis: co-factor in other liver diseases. Hepatology 2005;42:5–13. [ Links ]
5 Farrell GC, Larter CZ: Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99–S112. [ Links ]
6 Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F: Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009;51:433–445. [ Links ]
7 Lupsor-Platon M, Feier D, Stefanescu H, Tamas A, Botan E, Sparchez Z, Maniu A, Badea R: Diagnostic accuracy of controlled attenuation parameter measured by transient elastography for the non-invasive assessment of liver steatosis: a prospective study. J Gastrointestin Liver Dis 2015;24:35–42. [ Links ]
8 Wang Y, Fan Q, Wang T, Wen J, Wang H, Zhang T: Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med 2015;8:17654–17663. [ Links ]
9 Wong VW, Petta S, Hiriart JB, Camma C, Wong GL, Marra F, Vergniol J, Chan AW, Tuttolomondo A, Merrouche W, Chan HL, Le Bail B, Arena U, Craxi A, de Ledinghen V: Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol 2017;67:577–584. [ Links ]
10 Andrade P, Rodrigues S, Rodrigues-Pinto E, Gaspar R, Lopes J, Lopes S, Macedo G: Diagnostic accuracy of controlled attenuation parameter for detecting hepatic steatosis in patients with chronic liver disease. GE Port J Gastroenterol 2017;24:161–168. [ Links ]
11 Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Ledinghen V: Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343–350. [ Links ]
12 Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P: Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013–1021. [ Links ]
13 Lupsor M, Badea R, Stefanescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A: Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis 2008;17:155–163. [ Links ]
14 Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, Boisson RC, Bosson JL, Guyader D, Renversez JC, Bronowicki JP, Gelineau MC, Tran A, Trocme C, De Ledinghen V, Lasnier E, Poujol-Robert A, Ziegler F, Bourliere M, Voitot H, Larrey D, RosenthalAllieri MA, Fouchard Hubert I, Bailly F, Vaubourdolle M: Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol 2012;56:55–62. [ Links ]
15 Jung KS, Kim BK, Kim SU, Chon YE, Chun KH, Kim SB, Lee SH, Ahn SS, Park JY, Kim DY, Ahn SH, Park YN, Han KH: Factors affecting the accuracy of controlled attenuation parameter (CAP) in assessing hepatic steatosis in patients with chronic liver disease. PLoS One 2014;9:e98689. [ Links ]
16 Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ: Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009;29:1500–1506. [ Links ]
17 Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, Badea R, Laffi G, Marra F, Mangia A, Pinzani M: Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 2013;58:65–72. [ Links ]
18 Berzigotti A, De Gottardi A, Vukotic R, Siramolpiwat S, Abraldes JG, Garcia-Pagan JC, Bosch J: Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS One 2013;8:e58742. [ Links ]
19 Caetano AC LJ, Gonçalves B, Soares JB, Gonçalves R, Rolanda C: Será uma refeição ligeira fator de erro na avaliação da dureza hepática por elastografia transitória? Um estudo prospectivo. Jornal Português de Gastrenterologia 2013;21:102–108. [ Links ]
20 Ratchatasettakul K, Rattanasiri S, Promson K, Sringam P, Sobhonslidsuk A: The inverse effect of meal intake on controlled attenuation parameter and liver stiffness as assessed by transient elastography. BMC Gastroenterol 2017;17:50. [ Links ]
21 Lemoine M, Shimakawa Y, Njie R, Njai HF, Nayagam S, Khalil M, Goldin R, Ingiliz P, Taal M, Nyan O, Corrah T, D’Alessandro U, Thursz M: Food intake increases liver stiffness measurements and hampers reliable values in patients with chronic hepatitis B and healthy controls: the PROLIFICA experience in The Gambia. Aliment Pharmacol Ther 2014;39:188–196.
22 Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW: Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359–1368. [ Links ]
23 Alvarez D, Orozco F, Mella JM, Anders M, Antinucci F, Mastai R: Meal ingestion markedly increases liver stiffness suggesting the need for liver stiffness determination in fasting conditions. Gastroenterol Hepatol 2015;38:431–435. [ Links ]
24 EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–264. [ Links ]
25 Castera L, Forns X, Alberti A: Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835–847. [ Links ]
26 Castera L: Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293–1302.e4. [ Links ]
27 Friedrich-Rust M, Zeuzem S: Reproducibility and limitations of transient elastography. Liver Int 2009;29:619–620. [ Links ]
28 Szinnai C, Mottet C, Gutzwiller JP, Drewe J, Beglinger C, Sieber CC: Role of gender upon basal and postprandial systemic and splanchnic haemodynamics in humans. Scand J Gastroenterol 2001;36:540–544. [ Links ]
29 Dauzat M, Lafortune M, Patriquin H, Pomier-Layrargues G: Meal induced changes in hepatic and splanchnic circulation: a noninvasive Doppler study in normal humans. Eur J Appl Physiol Occup Physiol 1994;68:373–380. [ Links ]
30 Roulot D, Czernichow S, Le Clesiau H, Costes JL, Vergnaud AC, Beaugrand M: Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol 2008;48:606–613. [ Links ]
31 Sporea I, Sirli R, Deleanu A, Tudora A, Curescu M, Cornianu M, Lazar D: Comparison of the liver stiffness measurement by transient elastography with the liver biopsy. World J Gastroenterol 2008;14:6513–6517. [ Links ]
Statement of Ethics
All rules of the local ethics committee (“Comissão de Ética para a Saúde do Centro Hospitalar São João / Faculdade de Medicina da Universidade do Porto”) were followed, preserving patient identity and confidentiality.
Disclosure Statement
The authors declare that they have no conflict of interest.
* Corresponding author.
Dr. Marco Silva
Department of Gastroenterology, Centro Hospitalar de São João
Alameda Professor Hernâni Monteiro
PT–4200-319 Porto (Portugal)
E-Mail marcocostasilva87@gmail.com
Received: November 24, 2017; Accepted after revision: March 5, 2018
Funding Sources
The authors declare that there was no source of funding.
Author Contributions
Marco Silva: study design, data collection, and drafting of the manuscript. Armando Peixoto: data collection of the manuscript and drafting of the manuscript. Pedro Costa Moreira: data collection of the manuscript and drafting of the manuscript. Ana Luísa Santos: data collection of the manuscript. Susana Lopes: study design and critical revision of the manuscript. Regina Gonçalves: critical revision of the manuscript. Pedro Pereira: critical revision of the manuscript. Hélder Cardoso: study design and critical revision of the manuscript. Guilherme Macedo: critical revision and final approval of the manuscript.














