Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.3 Lisboa June 2019
https://doi.org/10.1159/000491559
CLINICAL CASE STUDY
Pocket Creation Method of Endoscopic Submucosal Dissection to Ensure Curative Resection of Rectal Neuroendocrine Tumors
Dissecção Endoscópica da Submucosa pelo Método de Pocket para Assegurar Ressecção Curativa de Tumores Neuroendócrinos Retais
Ikuhiro Koboria, Yasumi Katayamaa,b, Tomoyuki Kitagawaa, Yo Fujimotoa, Ryosuke Ouraa, Koji Toyodaa, Yuumi Kusanoa, Shinichi Banc, Masaya Tamanoa
aDepartment of Gastroenterology, Dokkyo Medical University Saitama Medical Center, Koshigaya City, Japan; bEndoscopy Center, Dokkyo Medical University Saitama Medical Center, Koshigaya City, Japan; cDepartment of Pathology, Dokkyo Medical University Saitama Medical Center, Koshigaya City, Japan
* Corresponding author.
ABSTRACT
Purpose: Pancreatic/gastrointestinal tract neuroendocrine neoplasm (NEN) is divided into neuroendocrine tumor (NET) and neuroendocrine carcinoma (NEC) according to the grade of malignancy, and differences are seen in clinical prognosis. NET, and rectal NET in particular, is often treated endoscopically. Endoscopic mucosal resection (EMR) was previously the main intervention for rectal NET, but EMR with a ligation device (EMR-L) and endoscopic submucosal dissection (ESD) are now also used. However, complete resection with these therapies is not always achieved. The pocket creation method (PCM) is a safe ESD method for colon tumors that offers a high en bloc resection rate compared with conventional colonic ESD. We performed ESD using the PCM for rectal NET and evaluated the complete resection rate. Methods: We performed ESD using the PCM in 4 patients. This procedure was technically feasible in all patients. Results: Endoscopically, all cases were resected en bloc, and pathological complete resection was achieved in all cases. No complications such as perforation or delayed postoperative bleeding were encountered. Conclusions: PCM should be considered when treating NET of appropriate size.
Keywords: Endoscopic submucosal dissection, Neuroendocrine tumor, Rectum
RESUMO
Objectivo: As neoplasias neuroendócrinas (NEN) do tracto gastrointestinal e pâncreas são divididas em tumores neuroendócrinos (NET) e carcinomas neuroendócrinos (NEC), dependendo do seu grau de malignidade, com diferenças no seu prognóstico clínico. Os NET, em particular os retais, são frequentemente tratados por endoscopia. A mucosectomia (EMR) foi previamente o método endoscópico principal para a exérese de NET retais mas a mucosectomia com bandas (EMR-L) e a dissecção endoscópica da submucosa (ESD) são agora também utilizadas. Contudo, a ressecção endoscópica completa com estas técnicas não é sempre possível. O método de criação de pocket (PCM) é um método seguro da ESD para ressecção de tumores do cólon que oferece uma taxa alta de ressecção em bloco quando comparado com a ESD convencional do cólon. Realizamos ESD por PCM para NET retais e avaliamos as taxas de ressecção completa. Métodos: Realizamos ESD por PCM em 4 doentes. Este procedimento foi tecnicamente possível em todos. Resultados: Endoscopicamente, todas as lesões foram removidas em bloco e a ressecção patológica completa foi alcançada em todos os casos. Não se verificaram complicações. Conclusões: O método de PCM deve ser considerado no tratamento de NET retais de tamanho apropriado.
Palavras-Chave: Dissecção endoscópica da submucosa, Tumor neuroendócrino, Reto
Introduction
Since the first report by Oberndorfer in 1907 [1], the term “carcinoid” has been used for neuroendocrine tumor (NET) of the gastrointestinal tract. According to the 2010 revision of the World Health Organization classification [2], a pancreatic/gastrointestinal tract tumor with characteristics and phenotype of the endocrine system is called a neuroendocrine neoplasm (NEN), with further division into NET and neuroendocrine carcinoma (NEC) according to the grade of malignancy and clinical prognosis. NET is a well-differentiated tumor, heterogeneity and malignancy are relatively low, and endoscopic treatment is often used, particularly for rectal NET. Previously, endoscopic mucosal resection (EMR) was mainly performed for rectal NET [3], but stumps showing positive results for residual tumor cells, local recurrence, and tumor remnants were the most important problems [4].
EMR with a ligation device (EMR-L) [5, 6] and endoscopic submucosal dissection (ESD) [6] have been used to treat rectal NET and have been reported to be more useful than EMR [7, 8]. On the other hand, the pocket creation method (PCM) was reported by Hayashi et al. [9, 10] as an ESD method for colon tumor, and has been described as a safe technique that offers a high en bloc resection rate compared with conventional colonic ESD [11]. In addition, the characteristics of the procedure make this method useful to ensure pathological evaluation in the vertical direction. PCM thus seems to have strong potential as a method for resecting NET, which exists within the very thin submucosal layer between the NET and muscularis propria, as the deep part of the stump is easily exposed during the procedure. The present study performed ESD using PCM for rectal NET and evaluated the complete resection rate.
Case Description
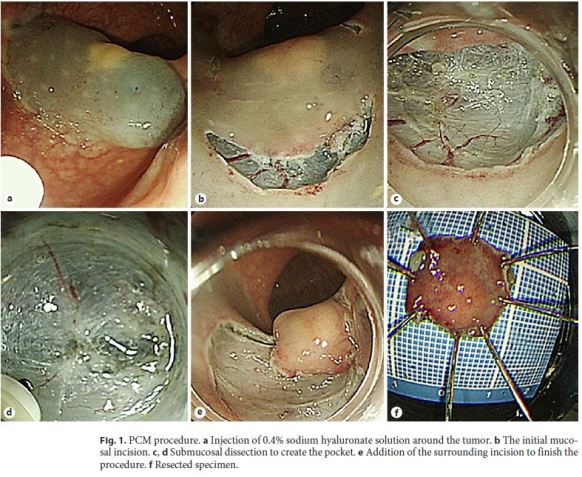
From November 2015 to February 2017, a total of 4 patients with rectal NET underwent ESD using PCM at Dokkyo Medical University Saitama Medical Center. The pathological complete resection rate was examined for cases in which ESD was performed using PCM for rectal NET. In addition, rates of complications such as perforation and delayed postoperative bleeding were examined. Lesions considered for endoscopic treatment were tumors less than 10 mm [12] that were restricted to the submucosal layer as evaluated by endoscopic ultrasonography and showed no distant metastasis/lymph node metastasis on computed tomography. For PCM, we used a LUCERA-ELITE video processor and GIF-260J videoendoscope (Olympus, Tokyo, Japan). We used an upper gastroscope for the PCM procedure, because this method offers better operability than colonoscope in the rectum. For endoscopic products, we used an MD-47951 SB soft hood (Sumitomo Bakelite, Tokyo, Japan) and a DualKnife electrosurgical knife (KD-650Q; Olympus). PCM was performed as previously described [9, 10]. Briefly, the initial mucosal incision was made after injection of 0.4% sodium hyaluronate solution. Submucosal dissection was performed to create the pocket. Finally, the remaining surrounding incision was added to finish the procedure (Fig. 1). This study was approved by our institutional review board.
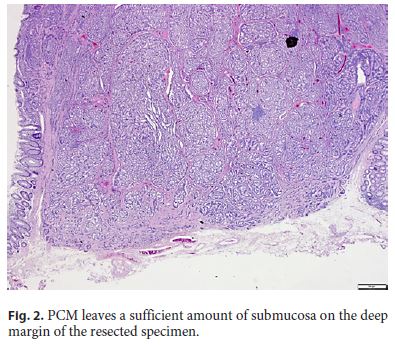
ESD using PCM proved technically feasible in all patients. Patient characteristics and technical results are shown in Table 1. The mean tumor size was 6.5 ± 2.1 cm, and the grade was G1 in all 4 cases. Endoscopically, all cases were resected en bloc, and histopathological examination indicated that complete resection was achieved in all cases. In particular, a sufficient amount of the submucosal layer remained for accurate pathological examination in the deep part of the tumor (Fig. 2). One case showed vascular invasion but has not shown any recurrence as of the time of writing. No complications such as perforation or delayed postoperative bleeding were encountered.
Discussion
In the endoscopic treatment of rectal NET, EMR treatment was initially mainstream. However, EMR often proved problematic in that pathologically confirmed complete resection was not achieved. EMR-L and conventional ESD techniques for rectal NET have represented advances, but incomplete resection is still often reported [13].
We likewise experienced inadequate results with EMRL and ESD, and several cases have required follow-up surgery. Moon et al. [14] reported that 7 of the 148 patients showed positive margins and therefore needed additional treatment. In patients with long-term follow-up with positive margins following endoscopic treatment without additional treatment, the local recurrence rate was 1.4% (2/141).
On the other hand, the PCM method reported in 2014 by Hayashi et al. [10] appears to be effective for laterally spreading tumors of the large intestine. Traction is important in ESD but difficult to apply in every situation. However, insertion of the transparent hood into the pocket in the PCM allows easy application of traction and countertraction [11]. PCM allows easy recognition of the muscular layer and thus placement of the incision just above this layer, facilitating dissection of the submucosal layer and reducing the rate of positive deep margins compared with EMR-L or conventional ESD. While PCM is more expensive and time-consuming than EMR-L, follow-up of a positive stump or incomplete resection is difficult and increases costs. Sufficient efficacy of the initial intervention is thus important and must be weighed against the potential economic and time costs of procedures for additional treatment and surveillance.
Although NET are not considered relatively low-grade tumors, NET with lymph node metastasis have the same prognosis as adenocarcinomas with metastasis [15]. Tumor diameter ≥10 mm and vascular invasion have been reported as risk factors for both lymph node metastasis and distant metastasis. According to the current consensus, adaptation of NET endoscopic treatment is considered to be warranted for lesions ≤10 mm in diameter [15]. A sufficient amount of the submucosal layer is necessary for accurate pathological examination, but resection of the tumor with a sufficient sample of submucosa is difficult in ESD or EMR-L. ESD by PCM can be performed leaving a sufficient amount of the submucosal layer on the deep margin side of the resected specimen, and the distance to the vertical stump can be secured.
In cases of NET with a small diameter, treatment can be performed simply and quickly using EMR-L. However, which treatment should be chosen to treat rectal NET according to tumor size remains unclear. If the distance to the vertical stump is considered difficult to secure because the tumor diameter is too large, ESD by PCM seems preferable. Proper use of PCM and EMR-L depending on the size of the tumor will therefore be a subject for future study. Interestingly, we observed vascular invasion in a 5-mm NET in in 1 of our patients. According to a report by Kitagawa et al. [16], addition of immunohistochemistry for D2-40 and Elastica van Gieson staining revealed lymphovascular invasion in 48.5% (32/66) of NET cases ≤5 mm in diameter. These included 1 case of lymph node metastasis, so careful follow-up is necessary for cases showing lymphovascular invasion. In conclusion, PCM should be considered when treating NET of appropriate size.
References
1 Modlin IM, Shapiro MD, Kidd M: Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol 2004;35:1440–1451. [ Links ]
2 Bosman FT, Carneiro F, Hruban RH, Theise ND (eds): WHO Classification of Tumors of the Digestive System, ed 4. Lyon, 2010. [ Links ]
3 Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K: Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy 1997;29:171–175. [ Links ]
4 Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, et al: Saline-assisted endoscopic resection of rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy 2004;36:202–205. [ Links ]
5 Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, et al: Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 2003;57:583–587. [ Links ]
6 Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, et al: Endoscopic submucosal dissection for rectal tumors. Endoscopy 2007;39:423–427. [ Links ]
7 Kim KM, Eo SJ, Shim SG, Choi JH, Min BH, Lee JH, et al: Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol 2013;37:275–282. [ Links ]
8 Zhong DD, Shao LM, Cai JT: Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis 2013;15:283–291. [ Links ]
9 Hayashi Y, Sunada K, Takahashi H, Shinhata H, Lefor AT, Tanaka A, et al: Pocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesions. Endoscopy 2014;46(suppl 1 UCTN):E421–E422. [ Links ]
10 Hayashi Y, Miura Y, Yamamoto H: Pocketcreation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumors. Dig Endosc 2015;27:534–535. [ Links ]
11 Sakamoto H, Hayashi Y, Miura Y, Shinozaki S, Takahashi H, Fukuda H, et al: Pocket-creation method facilitates endoscopic submucosal dissection of colorectal laterally spreading tumors, non-granular type. Endosc Int Open 2017;5:E123–E129. [ Links ]
12 Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, et al: Criteria for decision making after endoscopic resection of wall-differnciated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 2011;43:790–795. [ Links ]
13 Kaneko H, Hirasawa K, Koh R, Kobayashi R, Kokawa A, Tanaka K, et al: Treatment outcomes of endoscopic resection for rectal carcinoid tumors: an analysis of the resectability and long-term results from 46 consecutive cases. Scand J Gastroenterol 2016;51:1489–1494. [ Links ]
14 Moon CM, Huh KC, Jung SA, Park DI, Kim WH, Jung HM, et al: Long-term clinical outcomes of rectal neuroendocrine tumors according to the pathologic status after initial endoscopic resection: a KASID multicenter study. Am J Gastroenterol 2016;111:1276–1285. [ Links ]
15 Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H, et al: Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 2007;56:863–868. [ Links ]
16 Kitagawa Y, Ikebe D, Hara T, Kato K, Komatsu T, Kondo F, et al: Enhanced detection of lymphovascular invasion in small rectal neuroendocrine tumors using D2-40 and Elastica van Gieson immunohistochemical analysis. Cancer Med 2016;5:3121–3127. [ Links ]
Statement of Ethics
Informed consent was obtained from the patients for publication of this report. The ethics committee at Dokkyo Medical University Saitama Medical Center approved this study in accordance with the ethical guidelines of the 2013 Declaration of Helsinki.
Disclosure Statement
The authors have no conflicts of interest to disclose.
* Corresponding author.
Dr. Yasumi Katayama
Department of Gastroenterology and Endoscopy Center
Dokkyo Medical University Saitama Medical Center, 2-1-50 Minamikoshigaya
Koshigaya City, Saitama Prefecture 343-8555 (Japan)
E-Mail yasumi@dokkyomed.ac.jp
Received: March 2, 2018; Accepted after revision: May 21, 2018
Author Contributions
I.K. performed endoscopic therapy, Y. Katayama wrote and edited the manuscript, T.K. collected data, K.T., Y. Kusano, Y.F., and R.O. provided patient care, S.B. performed pathological studies, and M.T. edited the manuscript.














