Services on Demand
Journal
Article
Indicators
Related links
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.4 Lisboa Aug. 2020
https://doi.org/10.1159/000503455
CLINICAL CASE STUDY
Autoimmune Pancreatitis in na Adolescent: A Diagnostic Challenge
Pancreatite autoimune numa adolescente: um desafio diagnóstico
Rita Ramosa, Inês Carneirob, Rui Palmac, Paulo Calhaua
aDepartment of Paediatrics, Hospital Garcia de Orta, Almada, Portugal; bDepartment of Pediatrics, Hospital Santa Maria (CHLN), Lisbon Academic Medical Centre, Lisbon, Portugal; cDepartment of Gastroenterology, Hospital Santa Maria (CHLN), Lisbon Academic Medical Centre, Lisbon, Portugal
* Corresponding author.
ABSTRACT
Autoimmune pancreatitis (AIP) is a distinct subtype of pancreatitis, rare in the pediatric population. Its pathophysiology is poorly understood. A pancreatic focal mass is frequently the clinical presentation, which imposes the differential diagnosis with a pancreatic tumor. This distinction is essential because the treatment of pancreatic tumors is surgical in contrast to the treatment of AIP, which is pharmacological. We describe a case of a 16-year-old girl with AIP who presented with obstructive jaundice, weight loss, fatigue, and a pancreatic mass. This case emphasizes the importance of considering this diagnosis to correctly treat and prevent an abusive pancreatic resection.
Keywords: Pancreatitis, Jaundice, Adolescent
RESUMO
A pancreatite autoimmune (PAI) é um subtipo distinto de pancreatite, raro na população pediátrica, e com fisiopatologia ainda pouco conhecida. A apresentação clínica com massa pancreática é frequente, o que impõe o diagnóstico diferencial com neoplasia pancreática. A distinção entre estas duas entidades é fundamental uma vez que o tratamento dos tumores pancreáticos é cirúrgico, ao contrário da terapêutica farmacológica da PAI. Descrevemos o caso de uma adolescente de 16 anos diagnosticada com PAI, cujas manifestações clínicas foram icterícia obstrutiva, perda de peso, fadiga e massa pancreática. Realçamos a importância da suspeição e reconhecimento deste diagnóstico, para uma adequada intervenção terapêutica, que pode obstar a uma abusiva resseção pancreática.
Palavras-Chave: Pancreatite, Icterícia, Adolescente
Introduction
Autoimmune pancreatitis (AIP) is a rare autoimmune disorder that occurs primarily in adults and resembles pancreatic neoplasms.
It was first described by Sarles et al. [1] about 60 years ago but the term “autoimmune pancreatitis” was only introduced by Yoshida et al. [2] in 1995.
Adult AIP can be classified in two subtypes [2]. Type 1 AIP occurs predominantly in adults, is characterized by elevated serum IgG4 levels, is part of IgG4-related disease, and shows massive infiltration by IgG4 plasma cells on histology. Type 2 AIP presents in younger individuals, serological abnormalities are usually absent, and there are no systemic manifestations except for possible association with inflammatory bowel disease. The histology of type 2 AIP is characterized by neutrophilic infiltration, granulocytic epithelial lesions, and few, if any, IgG4 plasma cells. Pediatric AIP is a unique form of the disease with some similarity to type 2 AIP in adults. The first pediatric case was reported in 2008. However, to date, there are few pediatric case series described in the literature, and international recommendations for the approach to AIP have been released recently [3–6].
The differential diagnosis with pancreatic neoplasia is mandatory because the treatment of AIP is pharmacological and a correct and timely diagnosis can avoid an unnecessary pancreatic resection [7].
Owing to the rarity of this condition, we report a case of AIP which presented with jaundice and a pancreatic mass.
Case Report
A 16-year-old adolescent girl, previously healthy, presented with pruritus, asthenia, anorexia, and weight loss for 1 month, and jaundice for 3 days.
On admission, her physical examination was normal except for jaundice of the sclera and skin as well as lesions related to scratching. Initial laboratory studies showed total serum bilirubin 6.5 mg/dL, direct bilirubin 5.8 mg/dL, alkaline phosphatase 321 UI/L, γ-glutamyl transferase 33 UI/L, aspartate amino transferase 46 UI/L, alanine amino transferase 39 UI/L, lactate dehydrogenase 566 UI/L, and normal serum amylase; hemogram, erythrocyte sedimentation rate, and coagulation were normal.
Abdominal ultrasound revealed a prominence of the extrahepatic biliary tree with a distal echogenic agglomerate (11–12 mm). Magnetic resonance cholangiopancreatography (MRCP) showed a hypointense pancreas on T1-weighted images, and a solid mass (18 mm) in the head of the pancreas (Fig. 1) causing stenosis of the intrapancreatic choledochus and dilation of the upstream biliary tract (Fig. 2). Wirsung’s duct was not dilated and the remaining pancreatic parenchyma was normal.
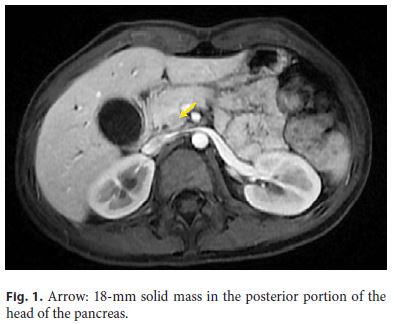
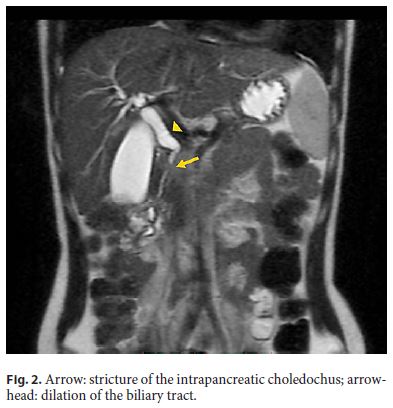
An endoscopic retrograde cholangiopancreatography (ERCP) confirmed the tight stricture in the distal third of the common bile duct. A plastic stent with a diameter of 7 Fr was placed, which led to clinical and analytical improvement. Common bile duct brushing and endoluminal biopsies were negative for neoplastic cells.
A transendoscopic ultrasonography (EUS) was performed. It confirmed that the plastic stent was in situ; however, it did not document either the biliary stenosis or the pancreatic head mass. In spite of the apparent normal ultrasound findings, FNA with a 25G × 1 needle was performed in the presumed location of the mass, based on image findings of ERCP and MRCP. The histopathological result showed inflammatory cells (lymphocytes and polymorphonuclear) and was negative for neoplastic cells.
During hospitalization, the patient underwent several analytical evaluations. Autoimmunity studies (antinuclear, anti-smooth muscle, antimitochondrial, anti-neutrophil cytoplasmic antibodies, and rheumatoid factor) were normal except for autoantibodies to thyroglobulin (normal thyroid function). Tumor markers (CEA, CA 19.9, and α-fetoprotein) were normal as well as serum IgG4.
Given the discordance of imaging findings between MRCP and EUS, a new MRCP was performed a month later and a similar result was noticed. At this point, according to clinical manifestations, imaging findings, and negative histopathological findings for neoplastic cells, we considered the hypothesis of AIP and tried a steroid trial to assist in a definitive diagnosis. The patient was treated with prednisolone (0.6 mg/kg/day) for 4 weeks with subsequent gradual dose reduction (5 mg/month).
Laboratory analysis normalized 2 weeks after the beginning of steroid therapy. The biliary stent had spontaneous mobilization and was eliminated. Serial MRCP showed a reduction of the pancreatic mass to 8 mm at 6 months and total remission at 15 months. At 18 months of follow-up she is asymptomatic, showing no signs of relapse, related complications, or symptoms suggestive of other autoimmune diseases.
Discussion
We describe a case of AIP in a previously healthy adolescent girl who presented with jaundice and a pancreatic mass. The diagnostic criteria for AIP in adults were postulated in 2011, based on pancreas histology, imaging findings, positive serology, the presence of other autoimmune or inflammatory organ diseases, and prompt response to corticosteroids [8]. In the adult population, two distinct forms of AIP are identified, with different histologic findings. Type 1 occurs predominantly in adults and is characterized by elevated serum IgG4 concentrations and IgG4-related extrapancreatic disease. Type 2 occurs in younger adults with normal IgG4 levels and no systemic manifestations, except for inflammatory bowel disease. Pediatric AIP is a unique form of the disease with some similarity to type 2 AIP in adults [4, 5].
Guidelines for the pediatric population were recently released and were not available at the time of clinical presentation, which made this case a diagnostic and therapeutic challenge [5].
The diagnosis of pediatric AIP, according to the recent guidelines, relies on the combination of clinical symptoms (usually abdominal pain, jaundice, weight loss, fatigue, and vomiting in contrast to painless jaundice in adults) and imaging findings, sometimes complemented with histopathology [4].
The initial presentation of our patient was jaundice, weight loss, and fatigue, but it was the pruritus that made the patient search for medical assistance. The differential diagnosis of jaundice in a female adolescent is wide and encompasses different diseases. The initial evaluation should differentiate conjugated from unconjugated jaundice. Conjugated hyperbilirubinemia is less common, but when present, the distinction between a hepatocellular and an obstructive cause is crucial [9]. Transabdominal ultrasound is an important first-line tool in this distinction; however, if AIP is suspected, an MRI/MRCP is necessary to support the diagnosis [5]. Although not specific of AIP, the MRI/MRCP findings are as follows: a focal, segmental, or global pancreas enlargement, a hypointense pancreas on T1-weighted images, a hypointense capsule-like rim on T2-weighted images, main pancreatic duct irregularities or stricture, and a common bile duct stricture or dilatation of the common bile duct which tapers toward an enlarged pancreatic head [5]. In contrast to adults, in the pediatric population, a focal pancreatic enlargement is more frequent than a diffuse one [4]. The presence of more than one imaging finding should raise the suspicion for AIP. In our case, a hypointense pancreas on T1-weighted images and a stricture in the common bile duct associated with a focal mass on the pancreas head were identified. The discordant imaging findings between MRCP and EUS may be justified by the poor quality of the ultrasound equipment used. In addition, if a more advanced ultrasound device were available, it would have been desirable that the EUS had been performed prior to the ERCP, to avoid the interference of the biliary stent.
Blood analysis may reveal an increase in lipase and amylase levels, as well as liver enzymes, in about half of the patients. Although IgG4 is a classic marker of AIP in adults (increased in 68–92% in AIP type 1 and 25% in AIP type 2) it is rarely positive in children, as happened in our patient [4].
A focal pancreas enhancement may be due to pancreatic malignant tumors (such as pancreatoblastoma or solid pseudopapillar epithelial neoplasms) or lymphoma. For this reason, a pancreatic biopsy is important not only to diagnose AIP but also to rule out cancer.
In adult studies, papilla biopsies seem to be less sensitive than EUS-guided pancreatic biopsies [5]. However, there are no available data for children. There are many barriers to EUS-guided pancreatic biopsies in the pediatric population. In fact, there are few pediatric endoscopists trained to perform EUS, there is little expertise in the interpretation of pediatric pancreatic histopathology, and it is difficult to obtain adequate pancreatic tissue with the currently available biopsy needles [5]. For this reason, and due to the fact that pancreatic cancer in children is extremely rare, recent pediatric guidelines suggest that diagnosis of pediatric AIP could be based on clinical and imaging findings [5].
In our patient, in order to rule out a neoplastic cause, an invasive procedure was performed before we could make the correct diagnosis. The histologic results often show lymphoplasmacytic infiltration, pancreatic fibrosis, and granulocyte infiltration [4]. These features were not identified in our case, probably due to the pathologist’s difficulty in interpreting pediatric pancreatic histopathology or less adequate material. The first option could have been overcome by revising the glass slide by a more experienced pathologist. The second MRCP was performed after knowledge of the histological result. Furthermore, at this point, the patient was clinically and analytically better, so we chose not to repeat the pancreatic biopsy. However, this could have been another possible approach.
A trial of steroid therapy can be performed before going through stent placement and biopsy, as the prompt response to corticotherapy is the hallmark of the disease [5]. Some studies reported cases with spontaneous regression; however, there are no comparative studies about complications and recurrence rates with and without treatment. Therefore, pediatric guidelines favor a course of prednisone [4, 5]. Our patient was medicated according to recommendations of adult guidelines (0.6–1 mg/kg/ day) and the weaning of corticosteroid therapy was then performed at a slower rate. Recent pediatric guidelines recommend a prednisolone dose of 1–1.5 mg/kg/day, so the pancreatic mass of our patient resolved with a lower dose of prednisolone. Therefore, further studies are needed to clarify the best therapeutic approach in AIP and to understand if the “wait and see” strategy is a real option.
Patients with AIP are at greater risk of developing other autoimmune or inflammatory diseases. They may progress to chronic pancreatitis and evolve exocrine and endocrine pancreatic insufficiency. Therefore, a regular followup is crucial to identify long-term complications [4, 5, 10].
References
1 Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreasan autonomous pancreatic disease? Am J Dig Dis. 1961 Jul;6(7):688–98.
2 Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality.
Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995 Jul;40(7):1561–8.
3 Blejter J, Weller S, Pace R, Cusumano H, Giambini D. Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg. 2008 Jul;43(7):1368–72.
4 Scheers I, Palermo JJ, Freedman S, Wilschanski M, Shah U, Abu-El-Haija M, et al. Autoimmune Pancreatitis in Children: Characteristic Features, Diagnosis, and Management. Am J Gastroenterol. 2017 Oct;112(10):1604–11.
5 Scheers I, Palermo JJ, Freedman S, Wilschanski M, Shah U, Abu-El-Haija M, et al. Recommendations for Diagnosis and Management of Autoimmune Pancreatitis in Childhood: consensus From INSPPIRE. J Pediatr Gastroenterol Nutr. 2018 Aug;67(2):232–6.
6 Kołodziejczyk E, Wejnarska K, Oracz G. Autoimmune pancreatitis in the paediatric population - review of the literature and own experience. Dev Period Med. 2016;20(4):279–86.
7 Martins C, Lago P, Sousa P, Araújo T, Davide J, Castro-Poças F, et al. Type 2 Autoimmune Pancreatitis: A Challenge in the Differential Diagnosis of a Pancreatic Mass. GE Port J Gastroenterol. 2017 Nov;24(6):296–300.
8 Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al.; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011 Apr;40(3):352–8.
9 Harb R, Thomas DW. Conjugated hyperbilirubinemia: screening and treatment in older infants and children. Pediatr Rev. 2007 Mar;28(3):83–91.
10 Friedlander J, Quiros JA, Morgan T, Zhang Z, Tian W, Kehr E, et al. Diagnosis of autoimmune pancreatitis vs neoplasms in children with pancreatic mass and biliary obstruction. Clin Gastroenterol Hepatol. 2012 Sep;10(9):1051–5.e1.
Statement of Ethics
All authors confirm that the adolescent and her parents gave written informed consent to publish this case.
Disclosure Statement
The authors have no conflicts of interest relevant to this article.
Funding Sources
All authors confirm that no funding was received for this study.
* Corresponding author.
Rita Ramos
Department of Paediatrics, Hospital Garcia de Orta
Avenida Torrado da Silva
PT–2805–267 Almada (Portugal)
E-Mail rita23ramos@gmail.com
Received: May 16, 2019; Accepted after revision: September 8, 2019
Author Contributions
Rita Ramos: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the manuscript, and revising the manuscript.
Ines Carneiro: acquisition of data, analysis and interpretation of data, drafting the manuscript, and revising the manuscript.
Rui Palma: acquisition of data, drafting the manuscript, and revising the manuscript.
Paulo Calhau: conception and design of study, acquisition of data, analysis and interpretation of data, drafting the manuscript, and revising the manuscript.
All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.














