Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.5 Lisboa out. 2020
https://doi.org/10.1159/00507199
RESEARCH ARTICLE
Epstein-Barr Virus Infection and Thiopurine Therapy in a Pediatric Population with Inflammatory Bowel Disease
Infecção pelo vírus Epstein-Barr e terapêutica com tiopurinas numa população pediátrica com doença inflamatória intestinal
Maria Do Céu Espinheira, Isabel Pinto Pais, Ivete Afonso, Jorge Ferreira, Eunice Trindade, Jorge Amil-Dias
Pediatric Gastroenterology Unit, Pediatric Department, Centro Hospitalar São João, Porto, Portugal
* Corresponding author.
ABSTRACT
Epstein Barr virus (EBV) primoinfection may contribute to the development of post-mononucleosis lymphomas in EBV-seronegative adult males with inflammatory bowel disease (IBD) under thiopurine therapy, but data on children are sparse. Knowledge of the EBV status may influence the type of surveillance and therapy in a group particularly vulnerable to the occurrence of EBV primoinfection. We aimed to determine the EBV status at diagnosis, the primoinfection rate, and complications in a pediatric Portuguese population with IBD. Method: This was a retrospective analysis of clinical records of pediatric patients with IBD. Demographic data, EBV status, as well as clinical and therapeutic data on primoinfection were collected. Results: Of the 250 patients evaluated, 229 (91.6%) had documented EBV screening and 50.8% were male. Mean age ± SD was 13.0 ± 2.8 years at diagnosis and 14.7 ± 2.3 years at EBV screening. EBV IgG serology was positive in 76.0% of patients. A total of 218 patients had been on therapy with azathioprine at some point. The average length of exposure to azathioprine was 4 years, and 91 patients (39.7%) were on azathioprine at EBV assessment. EBV primoinfection was documented in 4 patients (1.6%), all females, 2 of whom were on azathioprine. Two presented clinical signs of infection and 2 were identified at diagnostic screening; the first 2 suspended azathioprine and the other 2 did not initiate it. Conclusions: A significant proportion of pediatric Portuguese IBD patients are EBV-naïve. Systematic screening of EBV status enables the identification of patients at risk of primoinfection, and the occurrence of symptoms suggestive of acute EBV infection in seronegative patients should lead to rapid confirmation of the diagnosis. Timely diagnosis may allow the adjustment of therapeutic strategy sparing patients from potentially severe iatrogeny.
Keywords: Inflammatory bowel disease, Epstein-Barr vírus, Thiopurines
RESUMO
A primoinfecção pelo vírus Epstein Barr (EBV) pode contribuir para o desenvolvimento de linfomas pós-mononucleose infecciosa em homens adultos EBV-seronegativos com doença inflamatória intestinal (DII), sob terapêutica com tiopurinas. Os dados em crianças são escassos. O conhecimento do perfil serológico do EBV pode influenciar o tipo de vigilância e terapêutica em doentes particularmente vulneráveis à ocorrência de primoinfecção por EBV. Os autores têm como objetivo conhecer o perfil serológico do EBV ao diagnóstico de DII, a taxa de primoinfecção e complicações numa população pediátrica portuguesa com DII. Método: Análise retrospectiva dos processos de doentes em idade pediátrica com DII. Foram recolhidos dados demográficos, o perfil serológico do EBV e dados clínicos e terapêuticos aquando da primoinfecção. Resultados: Dos 250 doentes avaliados, 229 (91.6%) tinham rastreio do EBV documentado, 50.8% dos quais do sexo masculino. A idade média (DP) ao diagnóstico de DII foi de 13.0 ± 2.8 anos e no momento do rastreio do EBV de 14.7 ± 2.3 anos. A serologia para EBV foi IgG positiva em 76.0% dos doentes. 218 efetuaram terapêutica com azatioprina, com exposição média de quatro anos. 91 doentes (39.7%) estavam sob azatioprina aquando da avaliação do perfil serológico do EBV. A primoinfecção por EBV foi documentada em quatro doentes (1.6%), todos do sexo feminino, dois dos quais sob azatioprina. Dois doentes apresentaram sinais clínicos de infecção, dois foram identificados no rastreio infeccioso ao diagnóstico de DII; os dois primeiros suspenderam a terapêutica com azatioprina, os outros dois não a iniciaram. Conclusões: Uma proporção significativa de crianças portuguesas com DII é naïve para o EBV. A avaliação sistemática do perfil serológico do EBV permite a identificação de doentes com risco de primoinfecção e a ocorrência de sintomas sugestivos de infecção aguda pelo EBV em doentes soronegativos deve levar à rápida confirmação do diagnóstico. O diagnóstico oportuno permite o ajuste da estratégia terapêutica, evitando potencial iatrogenia grave.
Palavras-Chave: Doença inflamatória intestinal, Vírus Epstein-Barr, Tiopurinas
Introduction
Epstein-Barr virus (EBV) is a ubiquitous Human herpesvirus and seropositivity occurs in > 95% of the world’s adult population. It is rarely recognizable in children younger than 4 years of age, and at the age of 5 years only 20–25% of children in developed countries are EBV-positive [1]. Most EBV infections occur in adolescence and young adulthood, with rates ranging from 70 to 95% [2, 3]. Prevalence of EBV infection seems to be similar in inflammatory bowel disease (IBD) patients. A study from the Czech Republic evaluated children aged between 10.9 and 14.6 years, and found that 64% of patients were seropositive, with an increasing proportion of seropositivity during childhood [4]. A study of a cohort of Canadian IBD patients reported a prevalence of EBV seropositivity in the IBD population aged 18–25 years that was similar to that described in the general population, and a seropositivity approaching 100% in those > 25 years of age [5].
EBV is a lymphotropic microorganism and infected B cells are its primary reservoir in the body. In the acute phase of EBV infection, proliferating EBV-infected B cells are controlled primarily by natural killer (NK) T cells, T helper (CD4+) cells, and cytotoxic-suppressor (CD8+) NK T cells. Due to this T cell response, EBV-infected cell numbers drop significantly 4–6 weeks after acute infection and EBV remains in latent state inside B lymphocytes. In the presence of immunosuppressant conditions, EBV can reactivate the lytic phase and infect new cells, leading to the uncontrolled proliferation of infected lymphocytes and neoplastic cells [6, 7].
The most common EBV-associated lymphoproliferative disorder is B cell lymphoma, reflecting EBV affinity to B lymphocytes [8]. The association with posttransplant- like lymphomas caused by the reactivation of chronic latent EBV infection accounts for almost all thiopurine-related lymphomas in IBD adults > 30 years of age. The occurrence of Hodgkin and Burkitt lymphomas is also well known [9]. Another concern refers to early post-mononucleosis lymphoproliferation associated (or not) with hemophagocytic lymphohystiocytosis, reported exclusively in young male thiopurinetreated IBD patients with EBV primoinfection, and a rare but potentially fatal disorder more frequent in IBD patients on immunomodulators [10–12]. IBD patients are also at risk of developing hepatosplenic T cell lymphoma that occurs almost exclusively in males < 35 years who are exposed to thiopurines and biologic therapy for > 2 years.
In IBD patients, the risk of lymphoproliferative disorders seems to be similar or slightly higher than that in the general population and is related to the patient’s age and disease duration [8, 13, 14]. This can be potentiated by the use of certain drugs. Several case reports of lymphoproliferative disorders in IBD patients have been published, and the literature suggests that thiopurines are associated with a 4- to 5-fold increase in risk of lymphoproliferative disorders, particularly in men > 65 years of age [13–18]. The risk increases gradually for successive years of therapy, and there is evidence that discontinuing thiopurines reduces the risk of lymphoma [8, 17, 18]. Most of these cases of lymphoproliferative disorders in IBD patients on thiopurines were associated with EBV [15, 19].
The increased risk of lymphoproliferative diseases from EBV seroconversion in IBD patients on immunosuppressant drugs underlines the need for EBV serological monitoring in the EBV-naïve population. This seems to be most important in pediatric patients, a group particularly vulnerable to the occurrence of EBV primoinfection.
In this study, we analyzed EBV status in a pediatric Portuguese population with IBD, and the primoinfection rate, complications and therapeutic adjustments resulting from it.
Methods
This was a retrospective study of a pediatric population with IBD attending a pediatric gastroenterology clinic of a tertiary hospital over a period of 5 years (June 2013 to June 2018).
Medical records were reviewed. Variables analyzed included demographic data, duration of illness, treatment class and duration, EBV serology (VCA IgM and IgG, early antigen IgG, and EBNA IgG), PCR for EBV in cases of primoinfection and primoinfection rate. In cases of primoinfection, clinical and analytical data, treatment class at that moment, and follow-up of EBV status after primoinfection were recorded. Descriptive statistics were used.
Results
Clinical data of 250 children and adolescents with IBD were analyzed (60.8% had Crohn’s disease, 35.2% had ulcerative colitis, and 4.0% had unspecified IBD), with equitable distribution between genders (50.8% males). The mean age at IBD diagnosis was 13.0 ± 2.8 years (range 11 months to 18.3 years) (Table 1).
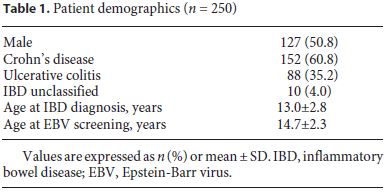
Of the 250 patients, 229 (91.6%) had EBV screening documented in the electronic medical records. Mean age ± SD at the time of EBV assessment was 14.7 ± 2.3 years; mean time ± SD between IBD diagnosis and EBV screening was 1.6 ± 1.8 years. In the last 3 years, 120 new cases of IBD were diagnosed and, in almost all of these, EBV screening was performed at the moment of diagnosis. There was a time gap between IBD diagnosis and EBV screening of 0.2 ± 0.3 years. Screening was performed in 32.4% of patients before initiating any treatment. EBV IgG was positive in 174 (76.0%) of the patients analyzed. All asymptomatic and seronegative patients were screened, and the screening was repeated when clinical symptoms suggested EBV infection.
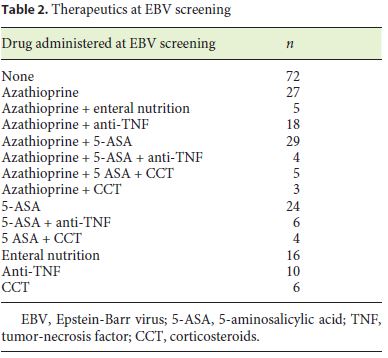
Two hundred and eighteen (87.9%) patients had been treated with azathioprine at some point of the disease course and 91 (39.7%) were on azathioprine at EBV assessment, either monotherapy or combined with other drugs (Table 2). Mean length of exposure to thiopurines was 4 years.
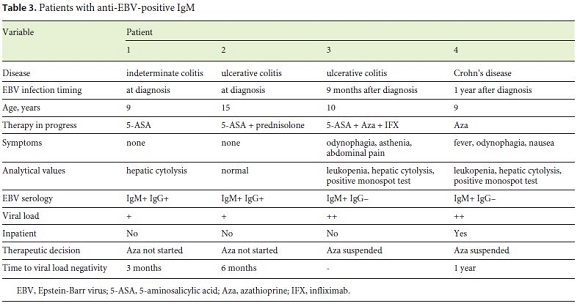
EBV primoinfection was documented in 4/250 (1.6%), all females aged 9–15 years. All presented EBV IgM and polymerase chain reaction positivity (mean viral load 91.1 × 103; between 2.5 and 239.0 ×103). Two presented clinical signs, namely fever, odynophagia, asthenia, nausea and abdominal pain; the diagnosis was confirmed in 1 week. In 2 others, the primoinfection was recognized at the diagnostic screening. One patient had hepatic cytolysis, 2 had both leukopenia and increased liver enzymes (maximum AST 429 U/L; maximum ALT 313 U/L). One patient needed hospitalization for symptomatic treatment and clinical surveillance. At the time of primoinfection, 2 patients were on azathioprine, either as monotherapy or in combination with anti-TNF therapy and 5-aminosalicylic acid, with a mean of 10 months between the initiation of thiopurine and documented EBV acute infection. In these 2 patients, azathioprine was suspended until the viral load was negative. The other patients did not initiate azathioprine therapy as a result of positive screening. The mean time until a negative viral load reached was 7 months (3–12 months) (Table 3).
In our cohort of patients, during this period, there were no cases of lymphoproliferative disorders.
Discussion
It is well recognized that primary EBV infection is associated with potentially fatal complications and lymphoma in patients with IBD who are being treated with thiopurines.
A large French prospective observational cohort study, CESAME, reported an independent association between ongoing thiopurine therapy and the risk of lymphoproliferative diseases; they found a hazard ratio of 5.28 for the development of lymphoproliferative disorders, despite an overall small risk, estimated in 1 lymphoma for every 300–1,400 years of thiopurine use. EBV was implicated, with a tendency for intestinal presentation. In patients on thiopurines, 12/15 lymphomas were posttransplant lymphoproliferative disorder (PTLD)-like (usually EBV-associated), and EBV primoinfection resulted in fatal infectious mononucleosis-associated lymphoproliferative disorders in 2 males [15]. Recently, Hyams et al. [20] published data from a prospective study of long-term outcomes of pediatric patients with IBD (the DEVELOP Registry), showing that thiopurine exposure was an important precedent event for the development of malignancy or hemophagocytic lymphohistiocytosis in these patients.
Routine screening for EBV virus status and adequate follow-up is still a controversial issue. There is no formal recommendation in the pediatric ECCO/ESPGHAN guidelines [21].
In our cohort, 91.6% had EBV screening documented at some point, with a mean interval between IBD diagnosis and EBV screening of 1.6 ± 1.8 years. In patients assessed in the last 3 years, the interval between IBD diagnosis and EBV screening was 0.2 ± 0.3 years, reflecting the rising concern about knowing EBV status before initiating thiopurines. In fact, currently, all patients have their EBV status determined at diagnosis.
We found a higher percentage of EBV IgG-positive (76.0%) patients in our cohort compared with other series of patients in the same age range. A study on a pediatric population from the Mount Sinai Medical Center reported that only 22% of their patients had a documented EBV status, 83% for asymptomatic screening and 52% who were checked before starting any treatment. In addition, 40% were IgG-positive [22], which is markedly lower than in our series.
The remaining 24.0% of our patients were EBV-seronegative and potentially susceptible to primoinfection. Such patients deserve special attention during follow-up, particularly if symptoms suggestive of active infection arise. In these cases, viral load quantification and serology should be performed. During infancy and early childhood, most EBV infections are asymptomatic, or present as mild disease indistinguishable from many other childhood infections. In adolescents and adults, EBV primoinfection is symptomatic in > 50% of cases, usually presenting as the classic infectious mononucleosis triad of fever, pharyngitis, and lymphadenopathy [2].
In our cohort, active EBV infection was documented in 4 patients (1.6%); 2 presented typical clinical signs and the other 2 were asymptomatic at primoinfection (which would not be recognized in the absence of systematic diagnostic screening performed at IBD presentation). In the patients on azathioprine at the time point of primoinfection, the treatment was suspended until the viral load was negative. In the patients identified from diagnostic screening, alternatives to azathioprine were administered until a negative viral load could be documented.
Presently, there is no specific treatment for EBV primoinfection. Aciclovir does not ameliorate the course of infectious mononucleosis in otherwise healthy individuals. Despite the lack of supporting evidence, in severe primary EBV infection, antiviral therapy with ganciclovir or foscarnet may be considered [23] and an investigation should be performed to exclude lymphoproliferative disorders. In some cases of EBV-associated lymphoproliferative disorders, a discontinuation of immunosuppressive therapy may be sufficient to induce spontaneous regression of the lymphoproliferative disease [24]. Clinical suspicion and EBV surveillance facilitate early recognition of primary infection, prompting adjustments in immunosuppressive therapy.
In our cohort, the EBV seroconversion rate during IBD treatment may have been underestimated as approximately 2/3 of patients did not undergo EBV screening at the time of IBD diagnosis.
Although the majority of reported cases of malignancy in IBD patients are reported in adults and its occurrence in children is rare, there are case reports on younger patients [16, 25, 26]. The risk of malignancy increases gradually for successive years of therapy, and there is evidence that discontinuing thiopurines reduces the risk of lymphoma [8, 17, 18]. In our patients, during this period, there were no cases of lymphoproliferative disorders.
A large percentage of our patients (87.9%) were treated with azathioprine. This reflects current therapeutic strategy and the expected severity of IBD disease in pediatric patients. It was also influenced by the fact that 60.8% of our patients had Crohn’s disease; in these patients, induction of remission with enteral nutrition is implemented and at the same time azathioprine is prescribed for maintenance.
Malignancy in IBD patients is a real concern but riskversus- benefit models suggest that benefits outweigh the risks in the case of thiopurine use [27]. Clinical surveillance should be ensured, always keeping in mind the possibility of a lymphoproliferative disorder.
In conclusion, a significant proportion of pediatric Portuguese IBD patients are EBV-naïve. Systematic screening of EBV status facilitates the identification of patients with a potential risk of EBV primoinfection as well as a timely adjustment of the therapeutic strategy, thereby reducing the risk of exposure to potentially severe iatrogeny.
References
1 Epstein MA. Reflections on Epstein-Barr virus: some recently resolved old uncertainties. J Infect. 2001 Aug;43(2):111–5.
2 Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010 May;362(21):1993–2000.
3 Crawford DH, Macsween KF, Higgins CD, Thomas R, McAulay K, Williams H, et al. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis. 2006 Aug;43(3):276–82.
4 Hradsky O, Copova I, Zarubova K, Durilova M, Nevoral J, Maminak M, et al. Seroprevalence of Epstein-Barr Virus, Cytomegalovirus, and Polyomaviruses in Children with Inflammatory Bowel Disease. Dig Dis Sci. 2015 Nov;60(11):3399–407.
5 Linton MS, Kroeker K, Fedorak D, Dieleman L, Fedorak RN. Prevalence of Epstein-Barr Virus in a population of patients with inflammatory bowel disease: a prospective cohort study. Aliment Pharmacol Ther. 2013 Nov;38(10):1248–54.
6 Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004 Feb;10(3):803–21.
7 Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001 Oct;1(1):75–82.
8 Vos AC, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, et al.; Initiative on Crohn’s and Colitis (ICC). Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis. 2011 Sep;17(9):1837–45.
9 International Agency for Research on Cancer WHO. Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Epstein-Barr virus and Kaposi sarcoma herpes virus/human herpes virus 8. Lyon, France, 17-24 June 1997. IARC Monogr Eval Carcinog Risks Hum. 1997;70:1–492.
10 Virdis F, Tacci S, Messina F, Varcada M. Hemophagocytic lymphohistiocytosis caused by primary Epstein-Barr virus in patient with Crohn’s disease. World J Gastrointest Surg. 2013 Nov;5(11):306–8.
11 Francolla KA, Altman A, Sylvester FA. Hemophagocytic syndrome in an adolescent with Crohn disease receiving azathioprine and infliximab. J Pediatr Gastroenterol Nutr. 2008 Aug;47(2):193–5.
12 Gidrewicz D, Lehman D, Rabizadeh S, Majlessipour F, Dubinsky M. Primary EBV infection resulting in lymphoproliferative disease in a teenager with Crohn disease. J Pediatr Gastroenterol Nutr. 2011 Jan;52(1):103–5.
13 Subramaniam K, D’Rozario J, Pavli P. Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: a review. J Gastroenterol Hepatol. 2013 Jan;28(1):24–30.
14 Sokol H, Beaugerie L. Inflammatory bowel disease and lymphopcroliferative disorders: the dust is starting to settle. Gut. 2009 Oct;58(10):1427–36.
15 Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, et al.; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009 Nov;374(9701):1617–25.
16 Barzilai M, Polliack A, Avivi I, Herishanu Y, Ram R, Tang C, et al. Hodgkin lymphoma of the gastrointestinal tract in patients with inflammatory bowel disease: portrait of a rare clinical entity. Leuk Res. 2018 Aug;71:1–5.
17 Khan N, Abbas AM, Lichtenstein GR, Loftus EV Jr, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013 Nov;145(5):1007–1015.e3.
18 Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015 May;13(5):847–58.e4.
19 Sokol H, Beaugerie L, Maynadié M, Laharie D, Dupas JL, Flourié B, et al.; CESAME Study Group. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012 Nov;18(11):2063–71.
20 Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, et al. Infliximab Is Not Associated with Increased Risk of Malignancy or Hemophagocytic Lymphohistiocytosis in Pediatric Patients with Inflammatory Bowel Disease. Gastroenterology. 2017 Jun;152(8):1901–1914.e3.
21 Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, et al.; European Crohn’s and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohn’s Colitis. 2014 Jun;8(6):443–68.
22 Gordon J, Ramaswami A, Beuttler M, Jossen J, Pittman N, Lai J, et al. EBV Status and Thiopurine Use in Pediatric IBD. J Pediatr Gastroenterol Nutr. 2016 May;62(5):711–4.
23 Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transplant. 2011 May;17(5):591–7.
24 Nissen LH, Nagtegaal ID, de Jong DJ, Kievit W, Derikx LA, Groenen PJ, et al. Epstein-Barr virus in inflammatory bowel disease: the spectrum of intestinal lymphoproliferative disorders. J Crohn’s Colitis. 2015 May;9(5):398–403.
25 Loganathan S, Zaitoun A, Charlton CP. Lymphoma with central nervous system involvement in a young patient with Crohn disease treated with azathioprine. J Pediatr Gastroenterol Nutr. 2010 Dec;51(6):790–2.
26 Destombe S, Bouron-DalSoglio D, Rougemont AL, Fournet JC, Ovetchkine P, Champagne J, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010 May;50(5):559–61.
27 Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000 Jun;118(6):1018–24.
Statement of Ethics
Ethics approval was not required because standard clinical surveillance data of the patients were used.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Maria Do Céu Espinheira
Pediatric Gastroenterology Unit, Pediatric Department, Centro Hospitalar São João
Alameda Prof. Hernâni Monteiro
PT–4200-319 Porto (Portugal)
Received: October 13, 2019; Accepted: December 27, 2019
Author Contributions
All authors contributed to the acquisition, analysis, and interpretation of the data for this work. E.T. and J.A.-D. contributed to work revision.














