Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.5 Lisboa Oct. 2020
https://doi.org/10.1159/000504762
CLINICAL CASE STUDY
Hepatic Myxoid Leiomyoma: A Very Rare Tumor
Leiomioma Mixoide Hepático: Um Tumor Muito Raro
João Fragaa, Rui Caetano Oliveiraa, Luigi Terraccianob, Mário Rui Silvaa, Maria Augusta Ciprianoa
aPathology Department, Coimbra University Hospital, Coimbra, Portugal; bPathology Department, Basel University Hospital, Basel, Switzerland
* Corresponding author.
ABSTRACT
Introduction: Mesenchymal tumors of the liver are rare, and in this group, myxoid leiomyomas are even rarer. So far, only 2 cases have been reported in the literature. Case Presentation: We aim to report the case of a 16-year-old female with a large lesion on the right hepatic lobe, grossly composed of gelatinous and heterogeneous tissue. Discussion: Histological evaluation revealed a benign mesenchymal neoplasm with expansive growth, paucicellular, with monotonous and dispersed spindle and ovoid cells, positive for α-smooth actin and h-caldesmon, without atypia or mitoses, consistent with the diagnosis of primary myxoid leiomyoma.
Keywords: Myxoid leiomyoma, Liver, Mesenchymal tumor
RESUMO
Introdução: Tumores mesenquimatosos do fígado são raros, e deste grupo os leiomiomas mixoides são ainda mais incomuns. Até ao momento foram apenas descritos dois casos na literatura. Caso clínico: Pretendemos efetuar a descrição de uma caso de uma menina de 16 anos com uma volumosa lesão no lobo hepático direito, que macroscopicamente era composta por um tecido gelatinoso e heterogéneo. Conclusão: Estudo histológico revelou uma lesão mesenquimatosa benigna de crescimento expansive, pouco celular, com células fusiformes e ovaladas, monótonas, com expressão de actina do músculo liso e h-caldesmon, sem atipia nem mitoses, consistente com o diagnóstico de leiomioma mixoide.
Palavras-Chave: Leiomyoma mixoide, Fígado, Tumor mesenquimatoso
Introduction
Primary liver cancer is the sixth most prevalent cancer worldwide, accounting for 5.7% of the overall incidence of cancer [1]. In 2018, the American Cancer Society estimated that liver and intrahepatic bile duct neoplasia corresponded to 4% of all new cases of cancer in men, with an estimated death rate of 6% among men and 3% among women [2, 3].
The most frequent types of neoplasia are epithelial, metastatic, or primary. Metastases to the liver are more common than primary tumors due to the dual blood supply of the liver from the portal and systemic circulation. Also, the hepatic sinusoidal epithelium has fenestrations which allow metastatic cells to penetrate more easily into the liver parenchyma.
Primary mesenchymal hepatic tumors vary from angiomyolipoma to synovial sarcoma. Primary leiomyoma of the liver is extremely rare; it is more commonly diagnosed in the uterus or gastrointestinal tract, but primary myxoid leiomyoma of the liver is an even rarer entity.
Case Presentation
Clinical Summary
A 16-year-old female was referred to our hospital due to a large lesion which occupied the entire right hepatic lobe. This lesion was accidentally discovered during an abdominal ultrasound on routine examination. No relevant pathological background was identified.
Pathological Findings
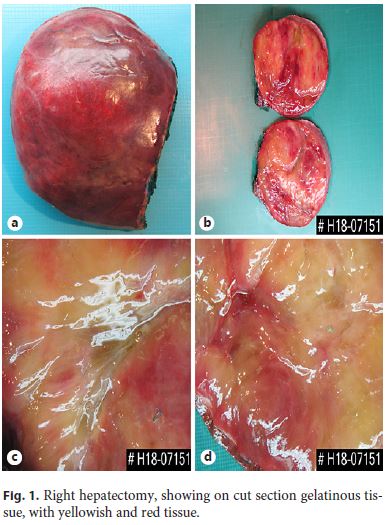
Right hepatectomy was made, and the surgical specimen weighed 2,086 g and was 25 . 16 . 9 cm wide, encompassing a surgical margin with 19 . 6 cm; it had a pink and smooth Gleason capsule (Fig. 1a) with a fibrotic retraction of the posterior face. In cut section, there was a large, well-defined, expansive tumor formation occupying the majority of the right lobe of the liver with a width of 15 . 11 . 19 cm that was largely located on the margin of the surgical resection. The tumor consisted of a translucent, gelatinous tissue of heterogeneous texture and color that varied between yellowish and pinkish, sometimes with hemorrhagic and soft-consistency areas (Fig. 1b, c). The interface with the normal parenchyma was perceptible, without any capsule surrounding the tumor, with brownish, homogeneous and soft liver parenchyma.
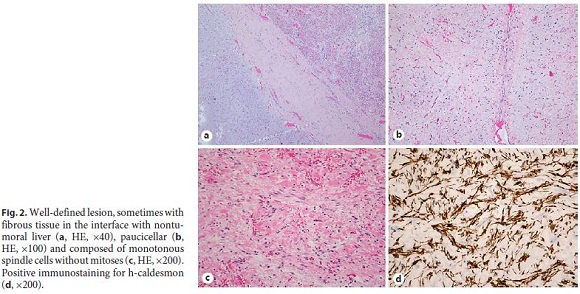
Histological evaluation revealed a benign mesenchymal neoplasm with expansive (Fig. 2a) and paucicellular growth (Fig. 2b), with monotonous and dispersed spindle and ovoid cells, with slightly eosinophilic cytoplasm and uniform nuclei, without atypia or mitoses (Fig. 2c). Stroma was markedly edematous and myxoid, well vascularized, with focal mononuclear inflammatory infiltrate. Bile ducts (CK7) or intratumoral hepatocytes (HepPar1) were not present. In the immunohistochemical study, spindle cells showed expression for α-smooth muscle actin and h-caldesmon (Fig. 2d), without staining for CD31, CD34, and desmin. Liver tissue adjacent to the neoplasia did not show any particular changes, nor did the margin of resection and the hilar structures.
Discussion
Primary mesenchymal tumors of the liver are very rare, accounting for less than 1% of all hepatic malignancies [1]. Hepatic angiosarcoma, leiomyosarcoma, and embryonal sarcoma are the most common mesenchymal tumors. The symptoms are unspecific with patients usually reporting abdominal pain, weight loss, weakness, loss of appetite, or vomiting. To these symptoms we may add enlargement of the liver, ascites, and jaundice, but these symptoms lack the specificity that would allow for a differential diagnosis between benign and malignant mesenchymal tumors. Therefore, it is difficult to make the diagnosis of malignant liver mesenchymal tumor because both its clinical presentation and imaging are nonspecific [4].
The main differential diagnoses encompass myxoma and mesenchymal hamartoma, the latter composed of loose connective tissue and epithelial bile duct or ductilelike in different proportions, with myxoid or collagenous stroma and arranged in a concentric way around the ducts [2]. Extensive tumor sampling is required in order to document the morphological spectra needed for the final diagnosis. In our case, the broad sampling provided the presence of spindle cells with smooth muscle differentiation required for the diagnosis and to rule out the presence of bile ducts and entrapped hepatocytes.
Primary myxoid leiomyosarcoma, rarely found in the liver, is also part of the differential diagnosis [5, 6]. However, there are no well-defined criteria for assessing the malignant potential of myxoid smooth muscle tumors outside the female genital tract [7]. Rubin and Fletcher [8] recommended this type of tumor to be considered as malignant when presenting any type of mitotic activity. Our tumor did not show necrosis nor mitosis.
Hawkins et al. [9] in 1980 established the criteria for the definition of hepatic leiomyomas, specifying that they should be composed of leiomyocytes and that there should be exclusion of leiomyoma in any other location, such as the uterus or gastrointestinal tract. The mean age of presentation was 43 years (range 5–87), with a mean size of 8.7 cm (range 2–30) and a female prevalence [10–12]. The review of 36 cases of hepatic leiomyomas done by Omiyale [13] in 2014 showed that 55.6% of the affected patients were women, with equal distribution between the right and left lobes. Imaging methods cannot differentiate between primary hepatic leiomyoma and other differential diagnoses. However, liver biopsy may be helpful [14].
The origin of the smooth muscle fibers from which these tumors are derived may be the walls of intravenous vessels, Ito cells (involved in myofibroblastic differentiation), or bile duct muscle cells or subcapsular mesenchymal cells [15–17], and the tumors may also be controversially associated with immunodeficiency states [10, 18]. The myxoid phenotype of these leiomyomas comes from the degeneration of muscle cells and increased interstitial matrix composed of mucopolysaccharide acid and glycoproteins [19]. Some cases are reported in association with Epstein-Barr virus infection in the context of immunosuppression, i.e., HIV infection or transplantation [20].
In short, we have described a very rare case of an extremely unusual mesenchymal neoplasia of the liver – myxoid leiomyoma – and the main differential diagnoses. As far as the authors are concerned, this is the third case reported in the literature [2, 15].
This case is important in order to increase awareness due to the rarity of the lesion. It may have a peculiar gross appearance, but clinical and radiological characteristics are unspecific, and larger tumors may give the impression of a malignant tumor. Histological study is fundamental and usually provides the right diagnosis.
References
1 Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006 Mar;23(1):047–63.
2 Choi HS, Jung CW, Cho SY, Kim SB, Park S. Primary myxoid leiomyoma of the liver. Korean J Pathol. 2014 Feb;48(1):54–7.
3 Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7–30.
4 Chen J, Du YJ, Song JT, e LN, Liu BR. Primary malignant liver mesenchymal tumor: a case report. World J Gastroenterol. 2010 Nov;16(41):5263–6.
5 Tsiatis AC, Atkinson JB, Wright JK, Cates JM. Primary hepatic myxoid leiomyosarcoma: a case report and review of the literature. Ultrastruct Pathol. 2008 Jan-Feb;32(1):25–8.
6 Tsuji M, Takenaka R, Kashihara T, Hadama T, Terada N, Mori H. Primary hepatic leiomyosarcoma in a patient with hepatitis C virus-related liver cirrhosis. Pathol Int. 2000 Jan;50(1):41–7.
7 Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006 Jan;48(1):97–105.
8 Rubin BP, Fletcher CD. Myxoid leiomyosarcoma of soft tissue, an underrecognized variant. Am J Surg Pathol. 2000 Jul;24(7):927–36.
9 Hawkins EP, Jordan GL, McGavran MH. Primary leiomyoma of the liver. Successful treatment by lobectomy and presentation of criteria for diagnosis. Am J Surg Pathol. 1980 Jun;4(3):301–4.
10 Prévot S, Néris J, de Saint Maur PP. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994;425(3):321–5.
11 Doyle H, Tzakis AG, Yunis E, Starzl TE. Smooth muscle tumor arising de novo in a liver allograft: A case report. Clin Transplant. 1991 Feb;5(1):60–2.
12 Perini MV, Fink MA, Yeo DA, Carvalho CA, Morais CF, Jones RM, et al. Primary liver leiomyoma: a review of this unusual tumour. ANZ J Surg. 2013 Apr;83(4):230–3.
13 Omiyale AO. Primary leiomyoma of the liver: a review of a rare tumour. HPB Surg. 2014;2014:959202. [ Links ]
14 Sousa HT, Portela F, Semedo L, Furtado E, Marinho C, Cipriano MA, et al. Primary leiomyoma of the liver: accurate preoperative diagnosis on liver biopsy. BMJ Case Rep. 2009;2009. pii:bcr09. [ Links ]2008.0898.
15 Yoon GS, Kang GH, Kim OJ. Primary myxoid leiomyoma of the liver. Arch Pathol Lab Med. 1998 Dec;122(12):1112–5.
16 Key RD, Rao RN. Leiomyomatous hemangiomas of the liver mimicking primary leiomyomas. Arch Pathol Lab Med. 1986 Jul;110(7):658–9.
17 Mahour GH, Wakim KG, Soule EH, Ferris DO. Structure of the common bile duct in man: presence or absence of smooth muscle. Ann Surg. 1967 Jul;166(1):91–4.
18 Ha C, Haller JO, Rollins NK. Smooth muscle tumors in immunocompromised (HIV negative) children. Pediatr Radiol. 1993;23(5):413–4.
19 Clement PB, Young RH, Scully RE. Diffuse, perinodular, and other patterns of hydropic degeneration within and adjacent to uterine leiomyomas. Problems in differential diagnosis. Am J Surg Pathol. 1992 Jan;16(1):26–32.
20 Wang MH, Wu CT, Hung CC, Liang JD, Chen PJ. Hepatic leiomyomatous neoplasm associated with Epstein Barr virus infection in an adult with acquired immunodeficiency syndrome. J Formos Med Assoc. 2000 Nov;99(11):873–5.
Statement of Ethics
This work was performed according to the Ethical Standards of Centro Hospitalar e Universitario de Coimbra.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Rui Caetano Oliveira
Serviço de Anatomia Patológica, Centro Hospitalar e Universitário de Coimbra
Piso-3, Praceta Mota Pinto
PT–3000-075 Coimbra (Portugal)
E-Mail ruipedrocoliveira@hotmail.com
Received: September 28, 2019; Accepted after revision: November 7, 2019
Acknowledgment
The authors would like to acknowledge Alexandra Costa for performing English review. The current work was not subject to any type of funding.
Author Contributions
J.F. and R.C.O. developed the manuscript and collected clinical data. M.R.S. contributed to the diagnosis and figure drafts. L.T. and M.A.C. supervised the work and added critical content to the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.














