A 44-year-old male presented with an 8-week course of progressively worsening bloating, dysphagia, regurgitation, vomiting, and weight loss (10 kg). He denied any other symptoms. He was diagnosed with metastatic non-small cell lung cancer 1 year before and was receiving pembrolizumab infusions every 3 weeks since then. Medication history included omeprazole, duloxetine, and pregabalin. He underwent an elective esophagogastroduodenoscopy that revealed a diffusely swollen and friable gastric mucosa from the cardia to the pylorus, covered with an off-white material due to exudate and sloughing mucosa along with scant food remains (Fig. 1a-c). Additionally, small patches of white exudate were identified in the duodenal bulb (Fig. 1d). Gastric and duodenal biopsies revealed a diffusely active gastroduodenitis with mixed inflammatory infiltration of the lamina propria and glandular distortion associated with ulceration of the mucosa (Fig. 2a). Prominent intraepithelial lymphocytosis, crypt abscess foci, and apoptotic bodies were also seen (Fig. 2b). No evidence of intestinal metaplasia or dysplasia was found. Immunohistochemistry forHelicobacter pyloriand cytomegalovirus detection were negative. Checkpoint inhibitor-induced gastroduodenitis was then presumed. Pembrolizumab was withheld, and oral prednisolone was initiated on 1 mg/kg and maintained for 2 weeks, with a subsequent taper dose of 5 mg/week. Symptomatic improvement was seen within days after initiation. Esophagogastroduodenoscopy was repeated 12 weeks later, showing only mild endoscopic improvement. Thus, corticosteroids were reinitiated as previously. Esophagogastroduodenoscopy was repeated at 24 weeks revealing marked endoscopic and histopathologic improvement.
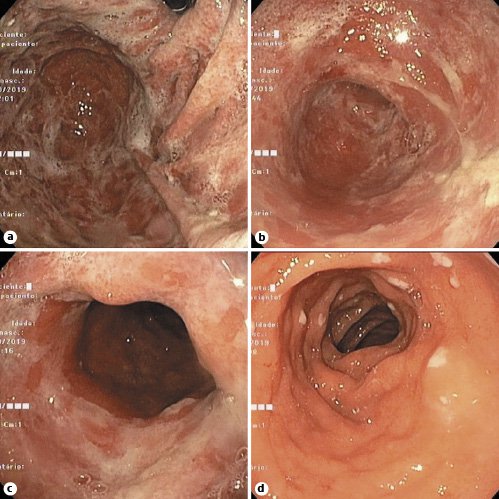
Fig. 1. Esophagogastroduodenoscopy revealing a diffusely swollen and friable gastric mucosa from the cardia to the pylorus (a-c), along with small patches of white exudate in the duodenal bulb (d).
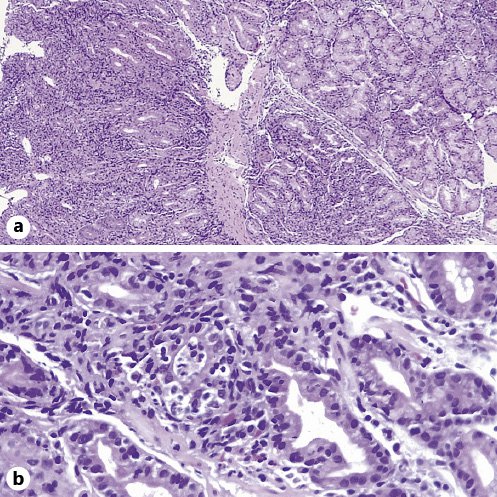
Fig. 2. Gastric biopsies revealing a diffusely active gastritis with mixed inflammatory infiltration of the lamina propria and glandular distortion associated with ulceration of the mucosa (a), along with prominent intraepithelial lymphocytosis, crypt abscess foci, and apoptotic bodies (b).
Immune-checkpoint inhibitors (ICI) are a class of immunotherapy drugs that lead to an antineoplastic immune response by blocking signalling via either the cytotoxic T lymphocyte antigen 4 (CTLA-4) pathway or the programmed cell death protein 1 (PD-1) pathway [1,2]. However, their use can lead to autoimmune-like side effects, designated as immune-related adverse events (irAEs) that can affect almost all organ systems [1,2]. Gastrointestinal toxicity, most commonly manifested by diarrhea, is the second most common irAE [1-3]. The upper gastrointestinal tract is rarely involved, and clinical manifestations include nausea, vomiting, dysphagia, and epigastric pain [1-3]. The onset of gastrointestinal irAEs usually occurs within 6-8 weeks after the initiation of therapy [1,3]. However, the occurrence of an irAE can occur at any point in time after the initiation of therapy, even after its discontinuation [3]. Unfortunately, there is still scarce data about gastrointestinal irAEs associated with anti-PD-1 antibodies, especially those related to upper gastrointestinal involvement [1,3]. Endoscopic findings are nonspecific and range from edema, erythema, and erosions to aphthous and deep ulcerations [3]. The differential diagnosis for ICI-induced gastritis includes infectious gastritis, idiopathic lymphocytic gastritis, and granulomatous gastritis [4]. A combination of diffuse, moderate-to-severe, chronic active gastritis with intraepithelial lymphocytosis and increased apoptotic activity appears to be the most helpful histopathological features for the diagnosis of ICI-induced gastritis [4]. Systemic corticosteroids should be used as the first-line therapy to manage inflammation, along with withholding the ICI [1,3]. In steroid-refractory patients the addition of infliximab or vedolizumab should be considered [1,3].
In conclusion, given the rapidly increasing use of these agents, it is of uppermost importance for gastroenterologists not only to be aware of potential gastrointestinal irAEs associated with ICI but also to be able to make an early recognition of these toxicities and appropriately manage them.














