Introduction
Drug-induced liver injury (DILI) is one of the most intriguing forms of liver disease due to the diversity of culprit substances and the variability of clinical presentations. Clinical trials are the first line for the evaluation of possible hepatotoxicity. Nevertheless, these are often underpowered to detect rare idiosyncratic toxicity. Most often, evidence is gathered through retrospective post-marketing pharmacovigilance studies [1]. The diagnosis of DILI requires the identification of a potential causative agent and exclusion of other forms of liver injury. That identification may be difficult, particularly in polymedicated patients. Although DILI is the most common cause of acute liver failure in the Western hemisphere, most cases are self-limited, resolving upon withdrawal of the trigger [2, 3].
Vedolizumab (VDZ) is a humanized antibody directed at α4β7 integrin. Inhibition of the interaction between this protein and mucosal vascular addressin cell adhesion molecule 1 (MaDCAM-1), hampers the migration of gut-homing CD4+ T lymphocytes, thus providing gut-selective immunosuppression. The effectiveness of VDZ has been proved in both clinical trials and real-world experiments, and it is approved for inductive and maintenance treatment of moderate-to-severe ulcerative colitis (UC) and Crohn’s disease [4, 5]. As far as the authors knowledge goes, there has only been one post-marketing case report of severe hepatoxicity attributable to VDZ [6]. Herein, we present the case of a patient presenting with acute hepatocellular pattern of liver injury probably related to VDZ.
Case Presentation
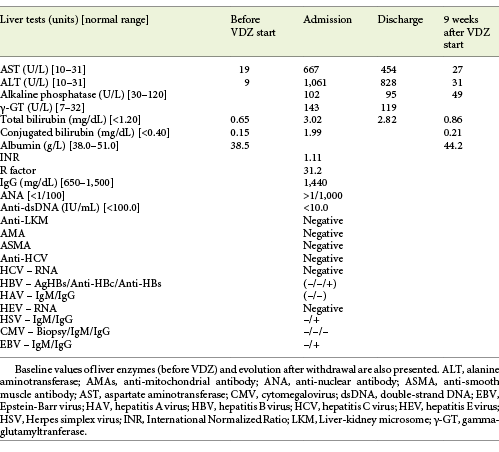
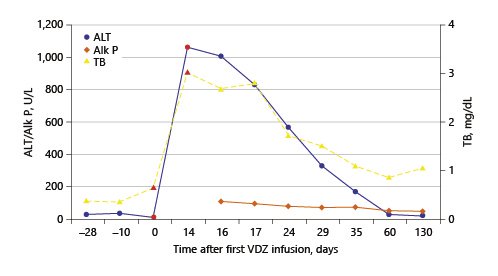
The authors present the case of a 41-year-old female followed at the gastroenterology outpatient clinic for UC diagnosed 11 years earlier, under treatment with oral salicylates (mesalamine 3 g/day) and oral budesonide (9 mg/day). Moreover, her medical record was remarkable for autoimmune hepatitis (AIH), diagnosed at the age of 31 years. Besides altered liver tests, she had increased IgG levels and positive anti-smooth muscle antibodies. Biopsy revealed lymphoplasmacytic portal tract expansion and centrolobular necroinflammatory lesions, as well as F2 stage of fibrosis (Fig. 1, 2). Pretreatment calculation of the revised original AIH score resulted in the diagnosis of definite AIH (score 20). Autoimmune hepatitis was first treated with budesonide; later the patient was started on glucocorticoids and azathioprine - the latter was withdrawn due to severe marrow suppression, and she remained effectively controlled with 10 mg of prednisolone. Additionally, she was taking lisinopril and simvastatin for hypertension and dyslipidemia, respectively. VDZ was added to her therapeutic scheme for UC because of frequent exacerbations under her previous treatment. She received two doses of VDZ on week 0 and 2. One day after the second dose, the patient presented to the emergency department with new-onset fatigue and painless jaundice. Beside jaundice, her physical examination was unremarkable. Laboratory results revealed elevated hepatic enzymes displaying a pattern of hepatocellular lesion (ALT elevated more than 30 times the upper level of normal; calculated R factor: 31.2; total bilirubin of 3.02 mg/dL). Pretreatment liver tests before starting VDZ were normal (Table 1). The patient was hospitalized for further diagnostic workup. She denied alcohol intake, drug abuse, use of herbal supplements or other over-the-counter drugs, recent travels, or high-risk sexual behaviors. Viral markers and autoimmune profile returned negative results (Table 1). Abdominal ultrasonography described hepatomegaly. At this point, the hypothesis of toxic hepatitis through VDZ-induced liver injury was deemed probable, attending to the chronologic sequence and absence of other apparent etiologies upon investigation. A percutaneous liver biopsy was then performed, demonstrating features of acute hepatitis overlapping chronic hepatitis, with centrolobular necroinflammatory activity, lymphoplasmacytic inflammatory infiltrate and moderate fibrosis (Fig. 3, 4), agreeing with the hypothesis of VDZ-induced acute hepatitis overlapping chronic autoimmune hepatitis. Causality was assessed through the application of the Council for International Organization of Medical Sciences (CIOMS) scale [7]; a score of 6 was obtained, compatible with probable relation between VDZ and hepatocellular damage (Table 2). The patient’s clinical evolution was favorable, and she was discharged 3 days after admission. VDZ was withdrawn after the two doses. No other changes were introduced to the patient’s usual medication. Subsequent repetition of liver tests exhibited normalization of aminotransferases over the following weeks (Fig. 5). The patient had no new UC flares, and endoscopic reevaluation 11 weeks after VDZ withdrawal revealed remitting UC.
Table 2: Council for International Organizations of Medical Sciences (CIOMS) causality assessment scale1 (adapted from Danan et al. [7])
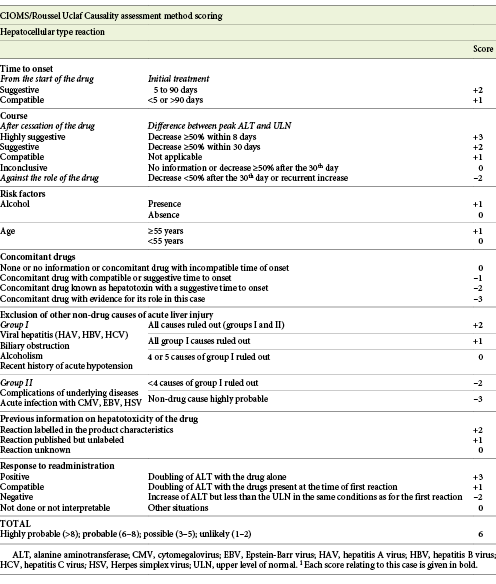
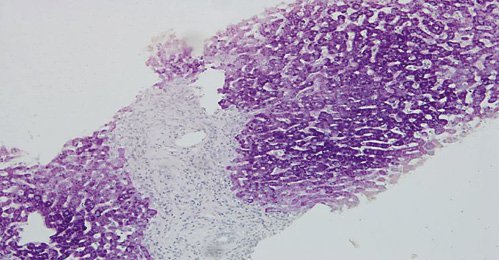
Fig. 1: Hematoxylin-eosin stain (×100) showing portal tracts expansion with polymorphic inflammatory infiltrate constituted by lymphocytes, plasma cells and eosinophils and low-density fibrosis.
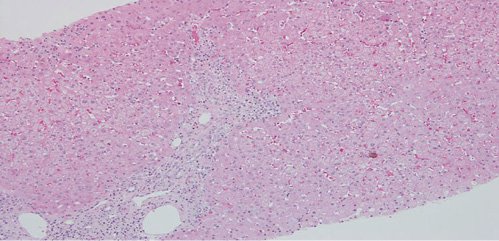
Fig. 2: Hematoxylin-eosin stain (×100). Multifocal intralobular necroinflammatory lesions, with rare foci of confluent necrosis. Small aggregates of macrophages revealing previous necroinflammatory activity.
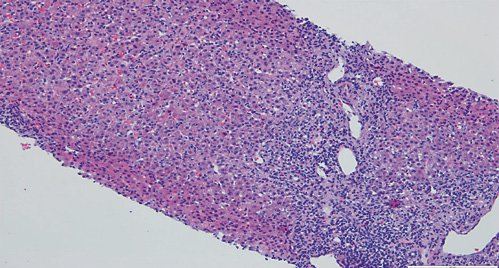
Fig. 3: Hematoxylin-eosin stain (×100) showing moderate fibrosis of portal tracts, with a polymorphic inflammatory infiltrate constituted by lymphocytes, plasma cells and eosinophils.
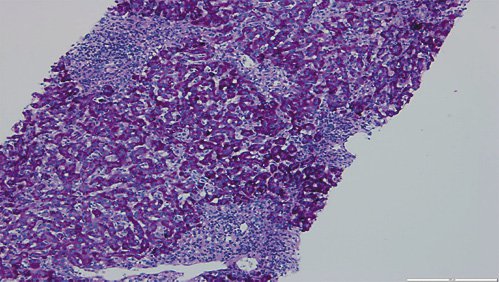
Fig. 4: Hematoxylin-eosin stain (×100) displaying parenchymal disarray, with swollen Kupffer cells and centrolobular necroinflammatory lesions. Some acidophil bodies are also seen.
Discussion/Conclusion
The case reported in this study constitutes, to the best knowledge of the authors, the first post-marketing case of a pattern of hepatocellular injury with probable relation to treatment with VDZ. The chronological relationship between the beginning of VDZ and the onset of hepatitis symptoms heightened our level of suspicion. Furthermore, an extensive workup for other causes of hepatocellular damage was undertaken and retrieved negative results, particularly viral markers, and autoimmune study (Table 1).
An important differential diagnosis to consider in this case was a flare of previously diagnosed AIH. Several factors pointed towards hepatotoxicity to VDZ instead of AIH: the patient had a clinically stable AIH controlled with a low dosage of prednisolone; the onset of liver test abnormalities after VDZ initiation; the patient was kept on her usual dosage of glucocorticoid, with complete normalization of liver tests upon withdrawal of the immunomodulatory therapy (Fig. 5). To further clarify our clinical suspicion, a liver biopsy was performed according to the recommendations of the European Association for the Study of the Liver (EASL) [1]. The biopsy revealed morphologic aspects of acute hepatitis caused by probable toxic insult, overlapping features of chronic liver disease. This case illustrates the importance of biopsy in the etiologic investigation of suspected toxic hepatocellular damage.
VDZ was the first gut-selective agent approved for the treatment of inflammatory bowel disease. Data regarding safety of VDZ collected from prelicensure phase 2 and 3 clinical trials report a lower adjusted incidence of adverse events (AEs) in VDZ-treated patients [8]. It is approved as a first-line biological therapy in UC [5]. Severe AEs occur in approximately 8% of the VDZ-exposed patients, but only 5% require withdrawal of the drug. Furthermore, the occurrence of AEs seems to be independent from the duration of exposure. Age-stratified analyses did not show an effect of age in the incidence of significant AEs [9]. The incidence of abnormal liver tests was not significantly different from placebo and did not lead to discontinuation of the drug. Elevation of ALT of more than 5 times the upper level of normal were rare (<2%) and occurred in similar frequency as in those under placebo [10]. However, Colombel et al. [8] described three cases of serious liver injury, two of them with probable AIH. Recent works, including data from both trial and post-marketing phases, confirm that hepatobiliary AEs are uncommon (<1% of all AEs registered), occurring in up to 5% of patients, and mostly related to abnormalities in the levels of liver enzymes [11, 12]. Stine et al. [6] reported the first post-marketing case of hepatotoxicity ascribed to the use of VDZ. Their case differed from ours in several aspects: first, their patient presented a cholestatic pattern of liver injury; second, their case presented after the third infusion of VDZ, and opposite to our patient, his symptoms developed later and more insidiously; third, their patient had persistent liver tests abnormalities 5 months after VDZ withdrawal, suggesting progression to chronic DILI. Similar to our report, their patient had a concomitant disease confounding the clinical picture (AIH in our report and primary sclerosing cholangitis in theirs). There was no evidence to support the role of any of these chronic diseases in the liver injury of both cases. Nevertheless, our study was complemented by a biopsy, which corroborated our hypotheses of VDZ-induced DILI.
This case highlights the necessary degree of suspicion for the diagnosis of rare events, such as clinically significant VDZ-induced hepatotoxicity. Moreover, it emphasizes the importance of a complete etiologic workup, particularly the role of liver biopsy, in order to exclude other differential diagnosis.