Dear Editor,
We present with great interest the follow-up of a case report published in the Portuguese Journal of Gastroenterology under the section Images in Gastroenterology and Hepatology(1).
A 44-year-old male undergoing immunotherapy with pembrolizumab for metastatic non-small cell lung carcinoma developed gastroduodenitis induced by an immune checkpoint inhibitor (ICI). He was treated with 2 consecutive courses of oral corticosteroids until endoscopic resolution. Treatment with pembrolizumab infusions was restarted after prednisolone had been completely tapered. After 3 cycles the patient presented dysphagia, bloating, nausea, and vomiting. He thus underwent an esophagogastroduodenoscopy which revealed a diffusely edematous and erythematous mucosa with friability and large patches of white coating, sugges-tive of relapse of pembrolizumab-induced gastritis as shown in Figure 1. The endoscopic findings were similar to the ones previously encountered. Histological examination excluded other etiologies, namely infection and neoplasia. Pembrolizumab was discontinued and prednisolone was initiated (1 mg/kg/day with a taper over 8 weeks), with symptomatic improvement within a few days. The patient is currently awaiting endoscopic reevaluation before a new pembrolizumab rechallenge.
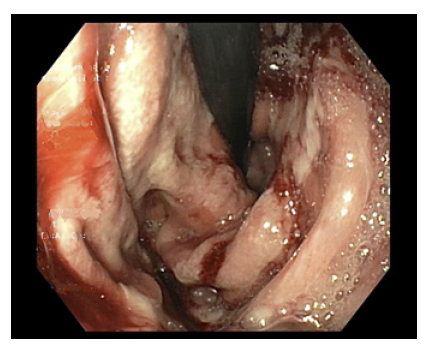
Fig. 1 Esophagogastroduodenoscopy after rechallenge with pembrolizumab. The gastric mucosa was diffusely edematous and friable, with extensive areas covered with white exudate.
Lower gastrointestinal (GI) immune-related adverse events (irAE) are well known toxicities of ICI; however, upper GI involvement is not commonly reported(2,3). Significant GI irAE are less frequent for anti-PD-1/L1 agents (up to 2%) than for CTLA-4 agents or combination regimens(2,3). The severity of the adverse events reported in this case was classified as grade 2 according to the National Cancer Institute CTCAE(4). There are no established guidelines for the management of upper GI tract irAE, and the generally adopted strategy for symptoms of grade 2 or higher is similar to that for colitis, i.e., withholding ICI administration and initiation of systemic corticosteroids or additional immunosuppressive agents for steroid refractory cases (e.g., infliximab or vedolizumab)(2,5,6). After appropriate management, the key point lies in when to restart ICI after an irAE, since it is of crucial importance for treatment and patient prog-nosis. However, limited data are available to support the clinician’s decision. Dolladille et al7 reported a recurrence rate of 28.6% for the same irAE after restarting the same anti-PD-1 monotherapy in patients with all types of cancer, but the recurrence rate seems to be lower for GI irAE.5,8Current guidelines recommend managing irAE based on their severity. Resuming PD-1/L1 agents is an option after grade 3 or lower grade adverse events, provided that patient can recover to grade 1 or lower. On the other hand, all ICI should be permanently discontinued in grade 4 toxicities(5). No guidelines currently support iterative rechallenge in patients with persistent recurrence of irAE.
Ultimately, the decision to restart ICI after an irAE must be discussed within a multidisciplinary team on an individual basis, taking into account the malignancy pro-gression/stability and the expected efficacy and potential risk of ICI rechallenge, along with appropriate monitoring and treatment of potential irAE.















