Introduction
Development of portal hypertension (PH) is a main cornerstone in the natural history of any chronic liver disease (CLD) regardless of the etiological cause and is responsible for the majority of complications [1]. Pathological increase in the portal venous pressure determined as the hepatic venous pressure gradient (HVPG) above the threshold of 10 mm Hg defines clinically significant PH and is associated with an increased risk of developing gastroesophageal varices (GEV) and overt clinical decompensation [2]. GEV develop at a rate of 7-8% per year in patients with compensated cirrhosis, and progression from small to large varices occurs at a rate of 10-12% per year, with decompensated cirrhosis being an independent predictor of progression [3]. Variceal hemorrhage (VH) occurs at a rate of around 10-15% per year and depends on the severity of liver disease, the size of varices and presence of red wale marks on endoscopy [3, 4]. Six-week mortality, which is recognized as the primary end point to assess the impact of therapies for acute VH, ranges between 15 and 25% [4]. If untreated, recurrent VH occurs in 60% of patients, usually within 1-2 years of the index bleed [3, 5]. In patients with GEV, an HVPG >12 mm Hg identifies bleeding risk, an HVPG >16 mm Hg indicates a higher risk of death, and an HVPG ≥20 mm Hg predicts failure to control bleeding, early rebleeding, and death during acute VH [5, 6]. Despite its excellent diagnostic and prognostic value, HVPG measurements require specific expertise, are invasive, relatively expensive, and available only in specialized centers.
There is consensus to perform esophagogastroduodenoscopy when the diagnosis of cirrhosis is established to screen for GEV and determine the care of varices [3]. Because endoscopy is a costly invasive procedure and may engender a large number of negative examinations, several authors have attempted to develop noninvasive reliable methods that could predict portal pressure so as to triage some patients [7]. The Baveno VI criteria were verified in several clinical studies to stratify high-risk esophageal varices (EV), and their use can obviate up to 20% of unnecessary endoscopies and merely 3-4% of patients with EV who need treatment would be missed [4, 8, 9]. The discriminative accuracy of noninvasive methods in predicting the presence of any GEV may be limited, although fairly accurate to rule out high-risk varices in patients with advanced CLD [10].
CD163 is a 130-kDa macrophage lineage-specific protein that acts as the scavenger receptor of the tight complex of hemoglobin-haptoglobin formed instantly in plasma when hemoglobin escapes red blood cells during intravascular hemolysis [11]. A soluble form of CD163 (sCD163) is present in the plasma and other body fluids, at least partly due to proteolytic shedding of the receptor from monocytes and macrophages [12]. The protein is constitutively released from the cells; however, in case of macrophage recruitment, the concentration increases acutely due to metalloproteinase-mediated cleavage near the cell membrane, and therefore sCD163 works as a specific biomarker of macrophage activation in various inflammatory diseases, such as hemophagocytic syndrome, sepsis, and liver diseases [12, 13]. Concentration of sCD163 was noticed to increase by 12% from the portal to the hepatic vein, which confirmed the hypothesis that sCD163 is mainly produced by the resident hepatic macrophages and can be viewed as a reflection of Kupffer cell activation in liver diseases [14]. Overexpression of CD163 in the blood and the liver has been demonstrated in viral and alcoholic hepatitis and acute liver failure [14-16]. In liver cirrhosis, sCD163 associates with liver disease severity scores, increases steeply with HVPG elevations, and accurately predicts disease progression [14, 17]. The rationale for using inflammatory serum biomarkers is based on the fact that PH is pathogenically related to liver injury and fibrosis, and that in turn these are associated with the activation of inflammatory pathways [18].
Therefore, the present work was designed to investigate the relation of serum sCD163, and other calculated noninvasive parameters, to the grade and bleeding risk of EV and the role for prediction of VH in patients with liver cirrhosis.
Materials and Methods
The present study included 100 patients with hepatitis C virus (HCV)-related liver cirrhosis who were referred to the Hepatobiliary Unit at Alexandria Main University Hospital. The diagnosis of liver cirrhosis was based on clinical, laboratory, and ultrasonographic findings. Also, 20 age- and sex-matched healthy subjects with no evidence of liver disease were included as control group.
Patients included in the study were divided into 3 groups. Group I (bleeder group) included 40 cirrhotic patients who presented with acute upper gastrointestinal bleeding (UGIB) proved to originate from EV on endoscopy. Group II (nonbleeder group) included 40 cirrhotic patients without any history of UGIB but revealed EV on surveillance endoscopy. Group III (no-varices group) included 20 cirrhotic patients without any history of UGIB and did not reveal any EV on surveillance endoscopy. Patients with PH were excluded from the study in cases of noncirrhotic PH, portal vein thrombosis or cavernomatosis, current use of noncardio-selective β-blockers, previous endoscopic variceal ligation or sclerotherapy, transjugular intrahepatic portosystemic stent placement, previous portosystemic shunt surgery, splenectomy or hepatectomy, serious infections or inflammatory diseases, any kind of malignancy like hepatic or esophageal cancer, or chronic diseases such as diabetes mellitus and cardiopulmonary or renal diseases.
All patients included in the study were evaluated clinically as regards age, sex, detailed history of UGIB, and manifestations of CLD. Routine laboratory investigations done for all patients and healthy subjects involved complete blood picture and liver test profile. Abdominal ultrasonography was done to assess echo texture of the liver and the presence of cirrhosis, the presence of ascites, and the splenic size that is taken as the maximal bipolar diameter from the inferior splenic tip to the superiomedial extremity. Severity of liver disease in patients with liver cirrhosis was identified according to the Child-Pugh class and score. Esophagogastroduodenoscopy was done for all patients to assess the presence of EV, their size and grade, the risk signs, and the stigmata of recent bleeding. EV were simply graded into small or large by quantitative size assessment with a suggested cutoff diameter of 5 mm, whereby large varices were those >5 mm in size [2]. The diagnosis of variceal risk signs is made when diagnostic endoscopy showed any of the following: red wale marks (defined as longitudinal dilated venules resembling whip marks on the variceal surface), or red spots (defined as localized reddish mucosal area or spots on the mucosal surface of a varix) [2, 3]. The diagnosis of VH is made when diagnostic endoscopy showed any of the following: active bleeding from a varix, a “white nipple” overlying a varix, clots overlying a varix, or varices with no other potential source of bleeding [2, 3]. Management of GEV and VH was made according to the clinical guidelines [3, 4].
Calculation of Noninvasive Parameters
Aspartate aminotransferase-to-platelet ratio index (APRI) calculated using Wai’s formula as (aspartate aminotransferase [IU/L]/upper limit of normal [IU/L]) × 100/platelet count (109 cell number/L) [19]
Fibrosis-4 score (FIB-4) calculated using Sterling’s formula as (age [years] × aspartate aminotransferase [IU/L])/(platelet count [109 cell number/L] × √ alanine aminotransferase [IU/L]) [20]
Platelet count-to-spleen diameter ratio (PSR) calculated as platelet count (109 cell number/L)/spleen diameter (millimeters) [21]
Measurement of Serum sCD163 Level
Quantitative determination of serum sCD163 levels in all patients and healthy subjects included in the study was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cat. No.: E-02073hu, Cloud-Clone Corp., Houston, TX, USA) according to the manufacturer instructions [22]. The serum sCD163 concentration was expressed as milligrams per liter.
Statistical Analysis
Data were fed to the computer and analyzed using the Statistical Package for Social Sciences (SPSS) software version 20.0. (Armonk, NY, USA; IBM Corp.). Quantitative data were described as range, mean ± SD, and median. Qualitative data were described as number and percentage. The Kolmogorov-Smirnov test was used to verify the normality of data distribution. Statistical significance of the obtained results was judged at the p < 0.05 level. All calculated p values were 2-tailed. The χ2 test and Fisher’s exact test with Monte Carlo correction were used for comparison between different groups with respect to categorical variables, as appropriate. Comparisons between 2 groups for normally distributed numerical variables were done using the Student t test. The Mann-Whitney U test was used to compare between 2 groups for nonnormally distributed numerical variables. Comparisons between more than 2 groups as regards normally distributed numerical variables will be performed by the one-way analysis of variance test with post hoc (Tukey’s) analysis. Comparisons between more than 2 groups as regards nonnormally distributed numerical variables will be performed by the Kruskal-Wallis test with post hoc (Dunn’s) analysis. Pearson’s correlation coefficient was used to measure the strength of association of the normally distributed numerical variables. Spearman’s correlation coefficient was used to measure the strength of association of the nonnormally distributed numerical variables. The receiver-operating characteristic curve was plotted to determine the cutoff value, sensitivity, specificity, and area under the receiver-operating characteristic curve (AUC) for serum sCD163 concentration and the other calculated noninvasive parameters to discriminate between the different patient groups. The positive predictive value (PPV) and negative predictive value (NPV) were calculated for the cutoff value which showed the highest sensitivity and specificity.
Results
Baseline clinical and biochemical data, calculated noninvasive parameters, and endoscopic findings of the groups of cirrhotic patients included in the study are presented concisely in Table 1.
Table 1 Baseline clinical and biochemical data, calculated noninvasive parameters, and endoscopic findings of the groups of cirrhotic patients included in the study
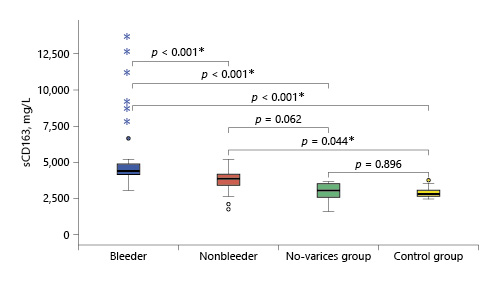
Serum sCD163 Level
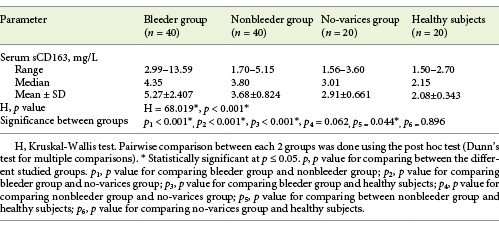
Serum sCD163 concentration ranged between 2.99 and 13.59 and 1.7 and 5.15 mg/L in cirrhotic patients of the bleeder group and the nonbleeder group, respectively, and it ranged between 1.56 and 3.6 mg/L in cirrhotic patients of the no-varices group, while it ranged between 1.5 and 2.7 mg/L in healthy subjects. The mean value of serum sCD163 levels showed a significant increase in all the groups of cirrhotic patients compared to healthy subjects (p < 0.001) with a stepwise increase among the no-varices group, the nonbleeder group, and the bleeder group sequentially (Table 2; Fig. 1).
Table 2 Statistical comparison between the groups of cirrhotic patients included in the study and healthy subjects as regards soluble CD163 (sCD163) serum levels (mg/L)
Statistical Correlation of sCD163 and the Other Calculated Noninvasive Parameters (APRI, FIB-4, PSR) to the Variceal Grade and Risk Signs
The mean value of serum sCD163 correlated positively with the grade of EV and the presence of variceal risk signs in the total sample of cirrhotic patients (p < 0.001 each) as well as in cirrhotic patients of both the bleeder group and the nonbleeder group (p = 0.002, p < 0.001 and p = 0.004, p < 0.001, respectively; Tables 3, 4).
Table 3 Statistical correlation of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to the variceal grade in the total sample and each group of cirrhotic patients included in the study
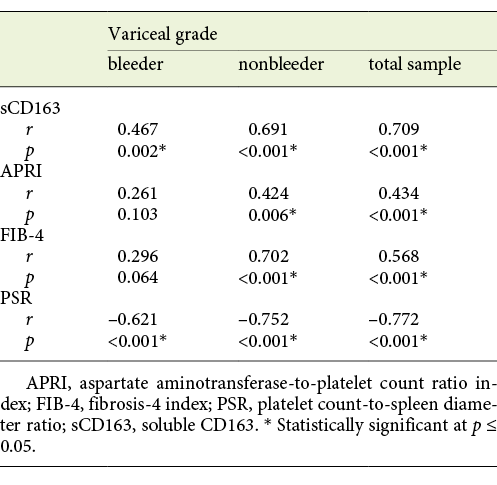
Table 4 Statistical correlation of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to the variceal risk signs in the total sample and each group of cirrhotic patients included in the study
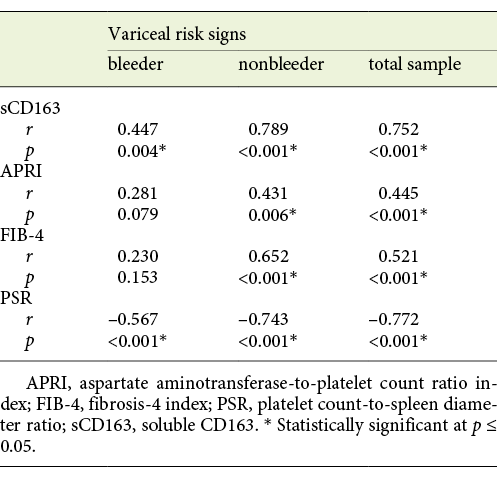
The mean value of APRI showed a positive correlation with the grade of EV and the presence of variceal risk signs in the total sample of cirrhotic patients (p < 0.001 each) and in cirrhotic patients of the nonbleeder group (p = 0.006 each) but not cirrhotic patients of the bleeder group (p = 0.103 and p = 0.079, respectively; Tables 3, 4). Also, the mean value of FIB-4 correlated positively with the grade of EV and the presence of variceal risk signs in the total sample of cirrhotic patients (p < 0.001 each) and in cirrhotic patients of the nonbleeder group (p < 0.001 each) but not cirrhotic patients of the bleeder group (p = 0.064 and p = 0.153, respectively; Tables 3, 4). However, the mean value of PSR correlated negatively with the grade of EV and the presence of variceal risk signs in the total sample of cirrhotic patients (p < 0.001 each) as well as in cirrhotic patients of both the bleeder group and the nonbleeder group (p < 0.001 each; Tables 3, 4).
Performance of sCD163 to Predict EV Presence in Cirrhotic Patients
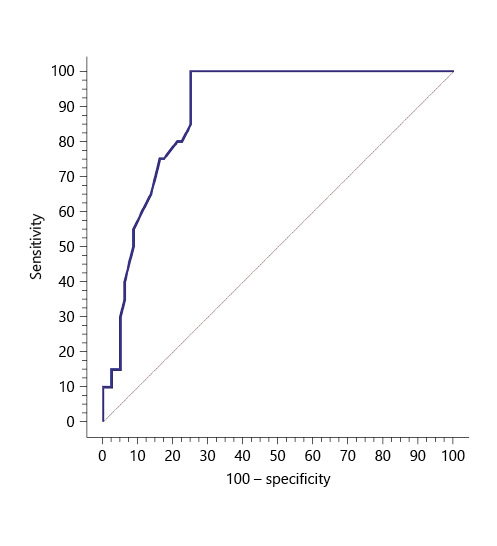
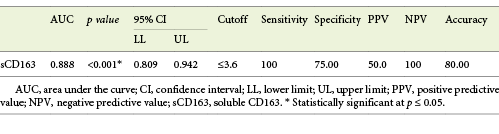
Serum sCD163 at the cutoff value of 3.6 mg/L significantly predicted EV presence in cirrhotic patients with 100% sensitivity and 100% NPV (AUC = 0.888, accuracy = 80%, p < 0.001) (Table 5; Fig. 2).
Table 5 Performance of soluble CD163 (sCD163) to predict EV presence in the total sample of cirrhotic patients included in the study
Performance of sCD163 and the Other Calculated Noninvasive Parameters (APRI, FIB-4, PSR) to Predict High-Grade and High-Risk EV in Cirrhotic Patients
Serum sCD163 at a cutoff value >4 mg/L yielded significant prediction of cirrhotic patients with large-size EV (AUC = 0.910, accuracy = 82%, p < 0.001; Table 6; Fig. 3) and high-risk EV (AUC = 0.939, accuracy = 87.8%, p < 0.001; Table 7; Fig. 4). Moreover, serum sCD163 at the same cutoff value significantly predicted the risk of index bleed in the nonbleeder group with 100% PPV (AUC = 0.977, accuracy = 95.4%, p < 0.001; Table 8; Fig. 5).
Table 6 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict high-grade esophageal varices in the total sample of cirrhotic patients included in the study
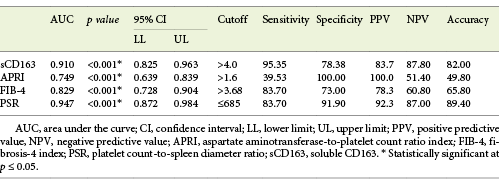
Table 7 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict high-risk esophageal varices in the total sample of cirrhotic patients included in the study
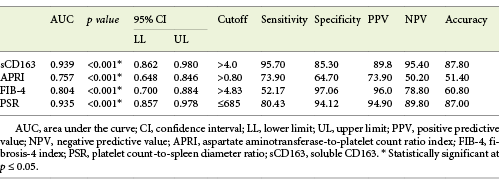
Table 8 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict the risk of index bleed in cirrhotic patients of the nonbleeder group included in the study
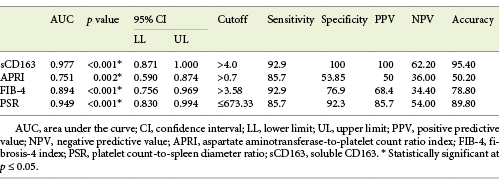
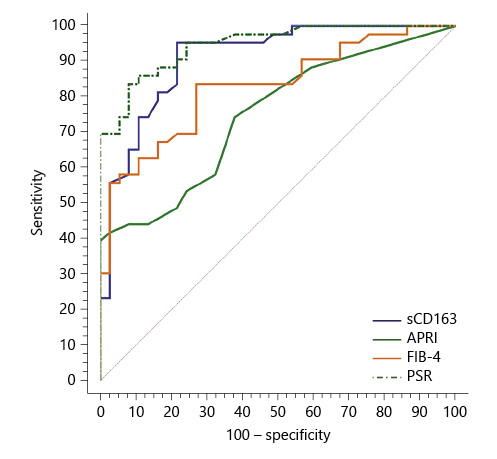
Fig. 3 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict high-grade esophageal varices in the total sample of cirrhotic patients included in the study.
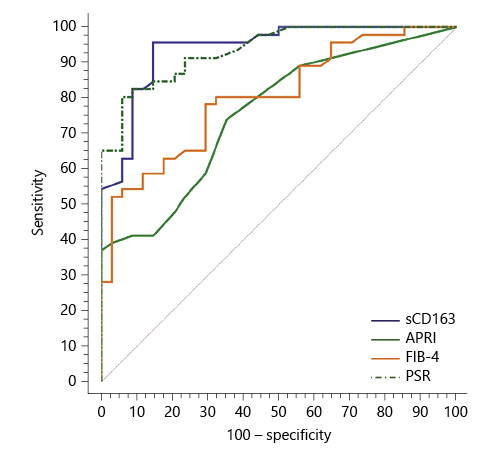
Fig. 4 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict high-risk esophageal varices in the total sample of cirrhotic patients included in the study.
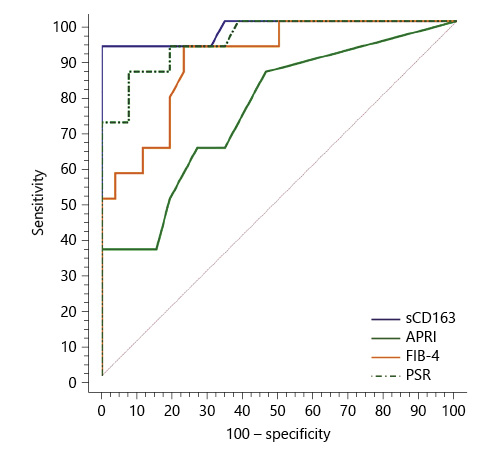
Fig. 5 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict the risk of index bleed in cirrhotic patients of the nonbleeder group included in the study.
All the other calculated noninvasive parameters performed significantly for prediction of high-grade and high-risk EV with considerable sensitivities and specificities. Amongst them, PSR at a cutoff value ≤685 had the best diagnostic performance in identifying cirrhotic patients with large-size EV (AUC = 0.947, accuracy = 89.4%, p < 0.001; Table 6; Fig. 3) and cirrhotic patients with high-risk EV (AUC = 0.935, accuracy = 87%, p < 0.001; Table 7; Fig. 4). Moreover, PSR at a cutoff value ≤673.33 significantly predicted the risk of index-bleed in the non-bleeder group with 85.7% PPV (AUC = 0.949, accuracy = 89.8%, p < 0.001; Table 8; Fig. 5).
Performance of sCD163 and the Other Calculated Noninvasive Parameters (APRI, FIB-4, PSR) to Predict VH Occurrence in Cirrhotic Patients
Serum sCD163 at a cutoff value >4.05 mg/L (AUC = 0.811, accuracy = 62.2%, p < 0.001) modestly discriminated cirrhotic patients with bleeding EV from cirrhotic patients with EV that had never bled yet with 85.3% NPV (Table 9; Fig. 6).
Table 9 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict variceal hemorrhage occurrence in the total sample of cirrhotic patients included in the study
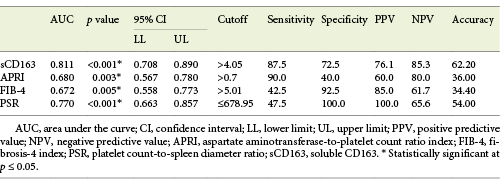
Fig. 6 Performance of soluble CD163 (sCD163) and the other calculated noninvasive parameters (APRI, FIB-4, PSR) to predict variceal hemorrhage occurrence in the total sample of cirrhotic patients included in the study.
PSR at a cutoff value ≤678.95 (AUC = 0.770, accuracy = 54%, p < 0.001) had the best diagnostic performance among the other calculated noninvasive parameters in discriminating cirrhotic patients with bleeding EV from cirrhotic patients with nonbleeding EV with 100% PPV (Table 9; Fig. 6).
Discussion/Conclusions
PH is a key event in the progression of most CLDs and is responsible for the majority of consequences of liver cirrhosis [1]. Kupffer cells and recruited macrophages play important roles in infection, inflammation, cell death, and fibrogenesis seen in cirrhosis and are often linked to the development of PH-related complications [23]. PH increases bacterial translocation and endotoxemia, which induces an inflammatory response in the liver and in the systemic circulation and increases portal venous pressure [24]. This vicious circle seems to be due to the endotoxin load leading to increased release of variable proinflammatory cytokines, activation of tumor necrosis factor-producing macrophages and monocytes, and subsequent coactivation of hepatic stellate cells leading to fibrosis [25, 26]. This sequence of events suggested that Kupffer cells, the fixed hepatic macrophages constituting the large majority of body macrophages, play an important role as a mediator between inflammation and PH [26]. CD163 is a macrophage lineage-related hemoglobin-haptoglobin scavenger receptor and a specific marker for macrophage activation [27]. The soluble form of CD163 is shed into the circulation after Toll-like receptor activation, and the serum concentrations of sCD163 are accordingly elevated during conditions of macrophage activation and proliferation [11, 28].
Patients with liver disease, such as hepatitis and cirrhosis, have high sCD163 levels which is probably related to the high number of activated Kupffer cells with strong CD163 expression in these conditions [14, 16]. Patients with acute hepatic failure have very high serum concentrations of sCD163, comparable with those in patients with macrophage activation syndrome, and a cutoff value of 26 mg/L identifies patients who are at high risk for mortality with a sensitivity and specificity of 62 and 81%, respectively [16, 29]. The concentration of sCD163 is highly increased in patients with liver cirrhosis, however, with a large variation among individual patients as related to the severity of the disease [30, 31]. Grønbaek et al. [17] reported that the circulating sCD163 concentration was nearly 3 times higher in cirrhotic patients than in controls. Even greater serum sCD163 levels compared to the values in healthy subjects were reported in other studies [14, 30]. In cirrhosis, serum sCD163 concentration is positively associated with the model for end-stage liver disease score and the Child-Pugh class, although the association with standard liver tests is weak or absent [14, 17, 30-32]. Moreover, cirrhotic patients who progressed from compensated liver disease showed a mean 2.5 times higher serum sCD163 concentration, and hence it could predict disease progression [30]. Interestingly, serum sCD163 was a strong predictor of overall survival in cirrhotic patients independently of the model for end-stage liver disease score, systemic inflammatory response, age, and gender [31]. These associations have important implications for using sCD163 as a prognostic marker in cirrhosis.
Interestingly, the plasma sCD163 concentration was linearly related to the portal venous pressure even after adjustment for cirrhosis status. This strong positive correlation to PH has been shown in 2 cohorts of cirrhotic patients and further confirmed in independent studies [14, 17, 31]. Grønbaek et al. [17] found that HVPG rose steeply to an asymptote of 22 mm Hg with increasing serum sCD163 up to 5 mg/L but not to higher values with higher sCD163 levels. A serum sCD163 cutoff value >3.95 mg/L (AUC = 0.83) predicted HVPG ≥10 mm Hg with PPV of 99%, yielding 66% sensitivity and 94% specificity. The biological explanation for such an association could be a direct involvement of Kupffer cells in the propagation of portal pressure by release of vasoactive substances and by propagation of fibrous tissue formation [17, 26]. Holland-Fischer et al. [14] found that Kupffer cells were activated in patients with liver cirrhosis in parallel with their PH; however, interestingly, the serum sCD163 concentration did not change after mechanical reduction of portal pressure by installation of a transjugular intrahepatic portosystemic stent. These findings suggested that Kupffer cell activation is a constitutive event which may play a pathogenic role for cirrhotic PH, and that sCD163, being a specific marker of activated macrophages, may independently predict HVPG and identify cirrhotic patients with clinically significant PH, but probably unsuitable for monitoring a reduction in portal venous pressure.
A pathological increase in the HVPG above the threshold of 10 mm Hg leads to the formation of portocaval shunts such as GEV with an increased risk of serious bleeding [3]. Supporting the relationship between sCD163 and portal pressure, a large Chinese study showed that the circulating sCD163 level was significantly elevated in cirrhotic patients complicated by EV compared to patients without EV (p = 0.015) [33]. A serum sCD163 cutoff value of 7.05 mg/L (AUC = 0.811) was good for predicting the presence of EV with 80% sensitivity and 89% specificity. Similarly, other studies found that the serum sCD163 level can distinguish cirrhotic patients having EV from those without varices with good sensitivities and specificities [14, 16, 17]. An Egyptian study found that the mean serum sCD163 level in cirrhotic patients with and without EV was increased fairly 3 times more than that of the control group and nearly doubled in patients with EV than patients without varices (p = 0.001); hence, it could potentially predict the presence of EV in Child-Pugh class A cirrhotic patients [34]. Another study found that serum sCD163 is a good noninvasive predictor for the presence of EV and may be used to determine the grade of varices [35]. It showed that the median serum sCD163 concentration was significantly elevated in cirrhotic patients with and without EV compared to healthy subjects (p = 0.009) and significantly higher in patients with large-size EV compared to patients with small-size varices (p < 0.001). This study suggested a cutoff value >191.71 ng/mL (AUC = 0.82) of serum sCD163 concentration to predict the presence of EV with a PPV of 86.1%, yielding 77.5% sensitivity and 75% specificity, and a cutoff value >199.19 ng/mL (AUC = 0.863) for the detection of large-size EV with a PPV of 89.5%, yielding 85% sensitivity and 90% specificity. Although another study reported that serum sCD163 cannot predict the presence of varices, its concentration was found to be significantly higher in patients with large-size EV (p = 0.012) and varices requiring treatment (p = 0.03); hence, it could serve as a good determinant of the grade of EV and the need for interventions [32].
In a cohort of cirrhotic patients, it was shown that patients with high serum sCD163 levels at baseline had a significantly higher risk of variceal bleeding in comparison to patients with a low concentration during follow-up, and a serum sCD163 level >4,100 ng/L was associated with VH independently of the variceal stage and red spots identified by gastroscopy [31]. Similarly, another study showed that serum sCD163 levels were significantly higher in patients at high risk of bleeding (p = 0.04), and the bleeder patients who experienced VH (p = 0.001) [32]. These studies suggested that plasma sCD163 could be a new independent noninvasive predictor for bleeding from EV in patients with liver cirrhosis.
Based on the results of the present work, the stepwise increase in serum sCD163 concentration in cirrhotic patients with varices and those who bled from EV compared to those who had never experienced UGIB suggests that the level of sCD163 expression could reflect the severity of PH. Also, the direct correlation of the serum sCD163 level to the grade of EV and the presence of variceal risk signs on endoscopy whatever the state of UGIB indicates that the serum sCD163 concentration could identify a subset group of cirrhotic patients who have varices needing treatment and those at risk of index bleed, so it could help decide on the need for doing endoscopy in order to plan for primary prophylaxis of varices in such cirrhotic patients. Hence; serum sCD163 is a potentially reliable simple noninvasive biomarker of PH, and its use could at least help refine the Baveno VI criteria. Unfortunately, the circulating sCD163 level performed modestly in discriminating cirrhotic patients with bleeding EV from those with EV that had never bled yet, and endoscopy would be required to make a definitive diagnosis of VH occurrence.
Noninvasive models such as APRI and FIB-4 were useful in predicting severe liver fibrosis or cirrhosis; however, it remains unknown whether these markers could identify patients without high-risk varices among those who do not meet the Baveno VI criteria [36, 37]. APRI was first introduced as a simple noninvasive test for the diagnosis of significant fibrosis and cirrhosis of various etiologies [19]. Subsequently, studies have shown that APRI correlated with HVPG, and an APRI of ≥1.09 had a diagnostic accuracy of 68% for predicting HVPG >12 mm Hg and hence could fairly predict the presence of EV [38, 39]. Sebastiani et al. [40] suggested APRI at a cutoff value of 1.4 for the prediction of EV and a cutoff value of 1.5 for the detection of large EV. Moreover, other studies proposed APRI at almost similar cutoff values for the prediction of EV [41-43]. However, Stefanescu et al. [44] suggested APRI at a cutoff value >2.201 (AUC = 0.538) for the detection of large EV. An Egyptian study reported that APRI at a cutoff value >1.26 (AUC = 0.695) could predict the presence of EV with PPV of 81.42%, and APRI at a cutoff value >1.47 (AUC = 0.734) could predict large EV [45]. Another study suggested a cutoff value of APRI >0.16 for the detection of EV and prediction of large EV [46]. Nevertheless, other studies found that the mean APRI was not significantly different between cirrhotic patients with small EV and those with large EV, and hence unable to predict the grade of EV, although it could identify varices [34, 35]. A retrospective study of a cohort of cirrhotic and noncirrhotic patients with acute UGIB evaluated APRI, among other noninvasive parameters, as predictors of VH [37]. For all patients with UGIB, APRI appeared to accurately predict the presence of varices prior to endoscopy and to be slightly less accurate in predicting a variceal culprit lesion as the cause of bleeding. For cirrhotic patients with UGIB, however, APRI did not distinguish between a variceal culprit and other lesions, and the optimal cutoff value useful for predicting varices as the culprit bleeding lesion could not be identified.
The FIB-4 score is a test derived from the Apricot database which produced interesting results as a good noninvasive marker of liver fibrosis in HCV-related CLD with performances similar to the Fibrotest [20]. FIB-4 was also tried for the prediction of EV in patients with liver cirrhosis [40, 44]. Sebastiani et al. [40] found that FIB-4 could fairly identify EV at a cutoff value ≥3.5 (AUC = 0.64), while the cutoff value ≥4.3 (AUC = 0.63) was good for the prediction of large EV. Stefanescu et al. [44] used FIB-4 for the diagnosis of EV at a cutoff value ≥3.98, while the cutoff value ≥6.75 performed well for the prediction of large EV. A much lower FIB-4 cutoff value at 2.8 was proposed in another study for predicting EV with a PPV of 92.7%, yielding 76% sensitivity and 80% specificity [47]. Nevertheless, other studies found that the mean FIB-4 was not discriminative between small and large EV, and hence unable to predict the variceal grade, although a high FIB-4 could potentially predict the presence of varices [34, 35]. Morishita et al. [48] included patients with HCV-related cirrhosis in a study to assess the clinical usefulness of acoustic radiation force impulse and other noninvasive parameters in the diagnosis of EV presence and risk. The acoustic radiation force impulse had the best diagnostic performance for predicting EV presence and identifying high-risk varices compared with APRI and FIB-4, although the serum-based parameters performed significantly. FIB-4 at a cutoff value of 6.21 (AUC = 0.745) and APRI at a cutoff value of 1.5 (AUC = 0.684) fairly diagnosed the presence of EV with acceptable performance. Moreover, FIB-4 at a cutoff value of 7.7 (AUC = 0.741) and APRI at a cutoff value of 1.62 (AUC = 0.669) significantly predicted high-risk EV with good accuracy.
The PSR is an easy-to-calculate index that initially showed an excellent performance for the prediction of EV at a cutoff value of 909, with NPV of 100% and PPV of 96%, and was confirmed to be reproducible even in the subgroup of patients with compensated disease [21]. Later, a multicenter study using the 909 ratio showed that the test performed less well than in the original study with PPV of 76.6% and NPV of 87.0% [49]. In validation studies, the test was less useful when compared with other noninvasive methods for EV prediction at different cutoff points [50-52]. Nevertheless, an Egyptian study reported a cutoff value of PSR at 939.7, which is very close to that reported by Giannini et al. [49], for prediction of EV presence in cirrhotic patients at 96.5% diagnostic accuracy [53]. Agha et al. [54] identified a PSR of 792 as the best cutoff value for the presence of EV in patients with compensated HCV-related cirrhosis and suggested that a greater ratio could be useful to identify patients at low risk of having EV on endoscopic surveillance. A modestly lower cutoff value was reported in other studies yielding good sensitivities and specificities for prediction of EV in cirrhotic patients, but it did not appear to predict the grade of varices [35, 55]. Excitingly, an Egyptian study used a cut-off value of 1,326.58 for the PSR to predict EV in HCV-related cirrhosis at 96.34% sensitivity and 94% diagnostic accuracy [56]. A meta-analysis of 20 studies calculated a sensitivity and specificity of 92 and 87% for the PSR, respectively, but there was a significant heterogeneity among the included studies, with some of them showing NPV as low as 43% [57].
A large cohort of Japanese patients with CLD were enrolled in a study to validate the clinical value of liver stiffness-spleen size-to-platelet ratio risk score and other noninvasive parameters for EV detection and identification of high-risk varices [58]. The liver stiffness-spleen size-to-platelet ratio risk score had the highest discrimination for EV presence and severity, although the other noninvasive parameters performed well. PSR at a cutoff value of 1,330 (AUC = 0.807) and FIB-4 at a cutoff value of 4.1 (AUC = 0.779) and APRI at a cutoff value of 1.2 (AUC = 0.749) significantly predicted the presence of EV at high diagnostic accuracies. Moreover, PSR at a cutoff value of 990 (AUC = 0.817) and APRI at a cutoff value of 1.7 (AUC = 0.762) and FIB-4 at a cutoff value of 5 (AUC = 0.716) fairly diagnosed high-risk EV with good performance.
In a cross-sectional study of Indian patients with alcoholic cirrhosis, Kothari et al. [59] found that only the PSR at a cutoff value <997 (AUC = 0.656) was significant for predicting EV with a diagnostic accuracy of 52.97%. Interestingly, PSR at a cutoff value <985 (AUC = 0.78) showed a good sensitivity of 81.97% with a diagnostic accuracy of 68.81% for the prediction of VH on follow-up. Also, FIB-4 at a cut-off value >3.91 (AUC = 0.74) performed well for the diagnosis of VH, yielding sensitivity of 72.13% and specificity of 60.28% with a diagnostic accuracy of 63.86%. Meanwhile, APRI at a cutoff value >1.05 (AUC = 0.72) showed lower sensitivity and specificity for the prediction of VH. This study suggested that PSR and FIB-4 may be the most useful among the armamentarium of noninvasive parameters for predicting the risk of VH in alcoholic cirrhosis. Nevertheless, in another study, Kraja et al. [60] found that none of the noninvasive parameters turned out to be a useful predictor of the index VH during the follow-up of nonbleeder patients with liver cirrhosis of variable etiologies.
Based on the results of the present work, the association of APRI, FIB-4, and PSR parameters to the grade of EV and the presence of variceal risk signs on endoscopy indicates that these noninvasive parameters could help identify cirrhotic patients with EV at a high probability of bleeding. Among the calculated noninvasive parameters, PSR yielded the highest discrimination for EV presence and severity, with acceptable predictability of VH in patients with liver cirrhosis.
In conclusion, the clinical usefulness of serum sCD163 solely or as a supplement to a panel of noninvasive approach to predict clinically significant PH and its major sequel of VH in cirrhotic patients could have an important future clinical implication with the objective to substitute for endoscopic surveillance and improve the guidelines for the prophylaxis of EV and management of VH.
In order to correct for the limitations in the present work, it may be recommended that the validity of serum sCD163 to predict the bleeding risk of EV, the occurrence of VH, and even the variceal recurrence after endoscopic obliteration in cirrhotic patients should be extensively studied in prospective longitudinal studies of large-scale populations with inclusion of patients with etiologies of chronic liver disease other than HCV infection. Moreover, future research is imperative to fully explore the potential role of sCD163 in other clinical stages of CLD (like decompensat84d liver disease) and discover its potential contribution to liver disease progression and prognosis. Further studies to search for any significant role of sCD163 in the pathophysiology of other consequences of PH (like ascites and spontaneous bacterial peritonitis) should be done as well.