Introduction
Acute upper gastrointestinal bleeding (UGIB) is a common clinical emergency worldwide, with an estimated annual incidence of 61-78/100,000 persons [1]. It requires frequent hospital admission and carries a substantial morbidity and mortality. Although the annual incidence has been decreasing, its 30-day mortality remains high at up to 11% [2]. The most common causes of acute UGIB are nonvariceal (mainly peptic ulcer disease and mucosal erosive disease) [3, 4]. Esophagogastroduodenoscopy (EGD) is the gold standard for the diagnosis and treatment of UGIB and it is associated with a reduction of blood transfusion requirements and the length of stay [5]. Substantial improvements in the management of UGIB have been incorporated into clinical practice in the last decades; however, there is still much controversy regarding the optimal timing for performance of endoscopy. Performance of early EGD within 24 h of patient presentation with suspected nonvariceal UGIB (NVUGIB) and no contraindication for endoscopy has been proposed as a key quality indicator in the management of UGIB [6] and is recommended by several guidelines [7-12]. Previous systematic reviews have demonstrated that early endoscopy (within 24 h of hospital admission) improved risk stratification of patients, provided a reduction of the hospital length of stay, and increased the use of therapeutic endoscopy, although the impact on need for surgery and in-hospital mortality was variable [13, 14]. A latter study reported increased mortality in patients not submitted to early endoscopy [15].
Some guidelines suggest that EGD may be considered earlier, i.e., within 12 h of presentation, in patients with severe bleeding or high-risk features [7, 9, 11, 16, 17]. However, risk stratification of patients with UGIB in low-risk and high-risk bleeding is highly variable between studies and the role of urgent endoscopy remains controversial.
Lim et al. [18], in a retrospective analysis that included 934 NVUGIB patients, revealed that patients with higher-risk bleeding, defined by a Glasgow-Blatchford score (GBS) ≥12, had a significantly lower mortality rate when submitted to urgent EGD (within 13 h of hospital admission); those authors concluded that patients with higher-risk bleeding benefit from earlier endoscopy. However, in lower-risk bleeding the time to endoscopy was not associated with in-hospital mortality. Kumar et al. [19] revealed that urgent EGD in lower-risk bleeding (GBS <12) was a predictor of negative outcomes (composite of death, rebleeding, and surgical, radiologic, or endoscopic intervention). In another study, among lower-risk bleeding, EGD within 24 h was associated with a lower in-hospital mortality but not rebleeding [20]. Therefore, there is still much controversy regarding the optimal timing of endoscopy in selected patients with different bleeding risks, and evidence that can precisely identify patients who should undergo early endoscopy is not available.
The aim of this study was to evaluate the impact of performance of endoscopy within 24 h on NVUGIB outcomes and to compare it in patients with lower-risk versus higher-risk bleeding.
Materials and Methods
Patient Selection and Definitions
We performed a retrospective unicentric cohort study including all consecutive patients undergoing EGD in the gastroenterology department of a university-affiliated hospital for suspected NVUGIB, between January 2015 and December 2018. This study was conducted in a hospital with a 12-h daily/week endoscopy service for gastrointestinal bleeding. Suspected NVUGIB was defined as hematemesis, melena, or hematochezia with suspected bleeding from the upper gastrointestinal tract [21].
In the situation that more than 1 EGD was performed during the same admission, only the finding of the index EGD was included. Exclusion criteria were as follows: patients aged under 18 years with bleeding from esophagogastric varices identified during EGD, patients without evidence of UGIB on EGD but with lower gastrointestinal bleeding confirmed during colonoscopy, and patients with development of NVUGIB while already in-patients for another reason (in-hospital bleeding). Besides, patients without the necessary records and data, such as time of admission and EGD performance, were excluded from the analysis.
According to the recommendations and to the emergency department protocol of our hospital, all patients were started on intravenous proton pump inhibitors (PPI) as soon as a suspected diagnosis of UGIB was made. Following endoscopy, patients with high-risk endoscopic stigmata were continued on intravenous infusion of PPI for 72 h. Otherwise, patients received oral PPI, if needed, as indicated by endoscopic findings. Erythromycin was administered previous to EGD, in patients with clinically severe or ongoing active UGIB, according to ESGE recommendations [7]. The antithrombotic management policy was carried out in accordance with the protocol of the immunohemotherapy department of our hospital, which adopts measures according to recommendations, so that the patient is optimized for the endoscopy, without, however, delaying and conditioning the performance of the procedure and outcomes.
Patients’ demographic and clinical data, including age, gender, type of presentation of bleeding, history of syncope, comorbidities (namely arterial hypertension, type 2 diabetes, cardiac failure, ischemic heart disease, cerebrovascular disease, renal failure, hepatic disease, disseminated neoplasia, or any other major comorbidity), medication use (PPI, nonsteroidal anti-inflammatory drugs, antiplatelets, anticoagulants, steroids, and selective serotonin reuptake inhibitors), hemodynamic assessment at presentation by vital signs, mental status at admission, biochemical results at presentation (hemoglobin, platelets, urea, albumin, and international normalized ratio), timing of endoscopy, and endoscopic findings/diagnosis were collected. The Charlson comorbidity index was calculated as previously described [22]. Outcome clinical data, including length of hospital stay, admission to an intermediate or intensive care unit, need for intervention (endoscopic or surgical), need for transfusion, occurrence of 30-day rebleeding, in-hospital mortality, and 30-day mortality, were also collected.
Altered mental status was defined as a Glasgow Coma Scale ≤14 or a physician designation of “disoriented,” “lethargy,” “stupor,” or “coma.” Hemodynamic instability was defined as presentation with a combination of systolic blood pressure below 100 mm Hg and a heart rate above 100 beats/min. Out-of-hours admission was defined as admission to a hospital during the period between 8:00 p.m. and 8:00 a.m. Weekend NVUGIB referred to those presenting between 8:00 p.m. on Friday and 8:00 a.m. on Monday. Timing of endoscopy was defined as the period between presentation to the emergency department (hospital admission) for NVUGIB and performance of endoscopy. High-risk stigmata ulcer was defined as active bleeding or a nonbleeding visible vessel. The decision of transfusion was made by the attending physician, following local emergency department transfusion protocols. Therapeutic endoscopic intervention included one or more of the following hemostatic strategies: use of hemoclips, argon plasma coagulation, or multipolar electrocoagulation, with or without previous injection of epinephrine. Cyanoacrylate injection was used when previous modalities of hemostasis had failed. Patients for whom endoscopic hemostasis could not be achieved were referred for surgery. In-hospital mortality referred to death during the hospital stay from any cause. After discharge, patients were followed up for a minimum of 30 days and 30-day rebleeding and mortality were evaluated. Rebleeding was defined as hematemesis or blood passing from a nasogastric tube, melena, or hematochezia with accompanying laboratory (hemoglobin drop of >2 g/dL within 24 h) or vital sign changes (systolic blood pressure decrease to <100 mm Hg or heart rate increase to >100 beats/min) [19] occurring within 30 days of hospital admission.
Patient Stratification Risk
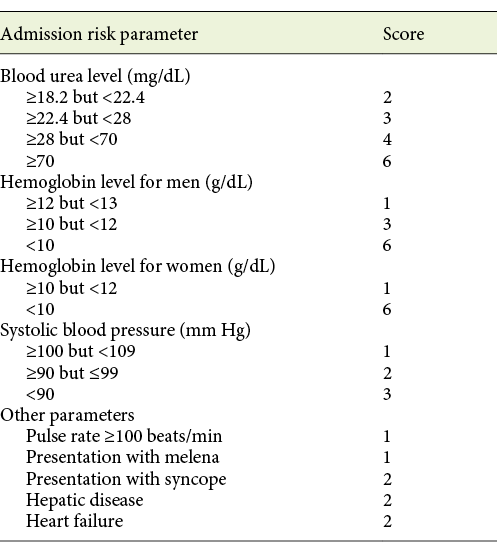
The GBS was assessed at admission and included the following nonendoscopic parameters: gender, systolic blood pressure, heart rate, presentation with melena, presentation with syncope, hepatic disease, cardiac failure, hemoglobin concentration, and blood urea nitrogen (Table 1) [23]. The patients were stratified by GBS as lower-risk (GBS <12) or higher-risk (GBS ≥12) bleeding according to the definition adopted in previous studies [18, 19, 24], whereas a cut-off of 12 demonstrated a specificity of 90% for prediction of all-cause in-hospital mortality in NVUGIB [18].
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software, version 26.0 (IBM, Armonk, NY, USA). Categorical data are presented as frequencies (%). Continuous normally distributed data are presented as means ± SD and continuous nonnormally distributed data are shown as medians (IQR).
A univariate analysis comparing clinical, laboratory, and outcome data of patients regarding the timing of endoscopy in the higher-risk and lower-risk bleeding groups was performed. Categorical variables were compared using the χ2 test or the Fisher exact test (2-tailed) as appropriate. Continuous variables were compared using the Student t test for independent samples or the Mann-Whitney test according to normal or nonnormal distributions of data, respectively. Measures of the effect size (g of Hedges, OR, and 95% CI) were calculated to quantify the magnitude of the differences found between the groups, thus evaluating the statistical power of the results.
A multivariate analysis of the association between timing of endoscopy and 30-day mortality, need for endoscopic treatment, and need for transfusion in lower-risk bleeding patients, adjusting for potential confounders, was performed by operating a binary logistic regression using the Enter method. The selection of potential confounders to include in the multivariate analysis was performed by considering parameters with statistical significance in the univariate analysis.
Two-sided p values <0.05 were considered statistically significant.
Results
Patient Characteristics
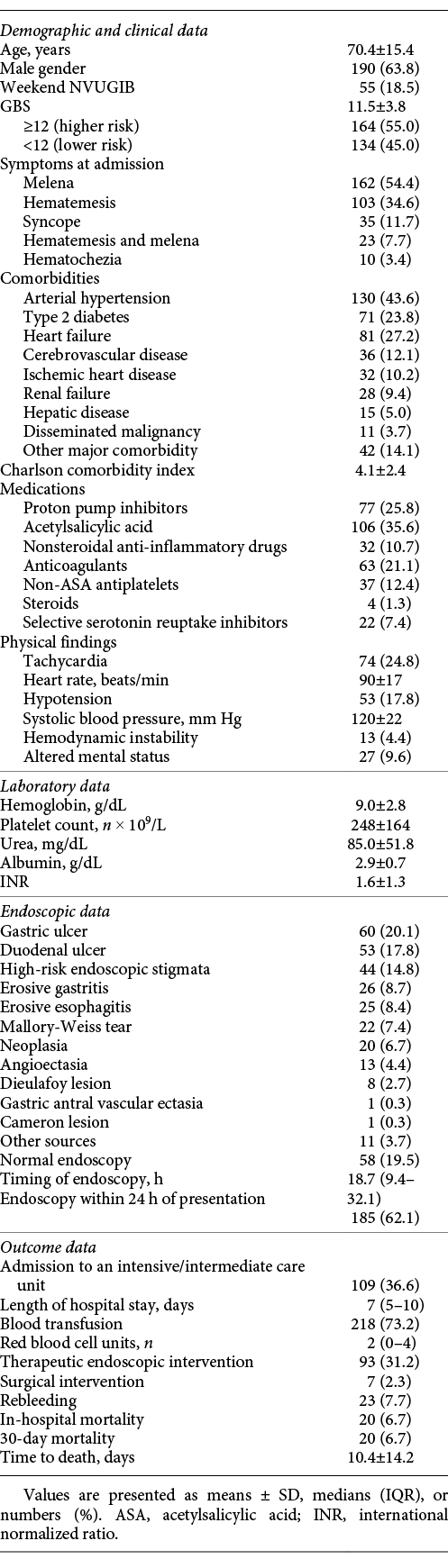
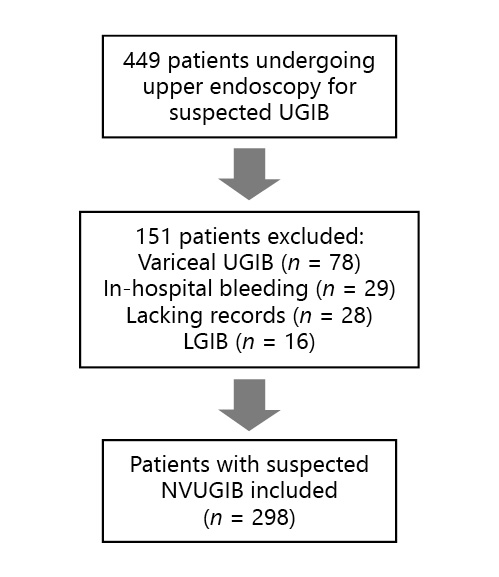
During the study period, 449 index EGD performed in our department for suspected UGIB were analyzed. According to the defined exclusion criteria, 78 patients with variceal bleeding confirmed at EGD, 16 patients with lower gastrointestinal bleeding confirmed at colonoscopy, 29 patients with in-hospital bleeding, and 28 without the necessary records were excluded. A flow chart illustrating the sampling process is presented in Figure 1. A total of 298 patients were included; 63.8% (n = 190) were male, with a mean age of 70.4 ± 15.4 years and a mean Charlson comorbidity index of 4.1 ± 2.4. Arterial hypertension and heart failure were the most common comorbidities, being present in 43.6% (n = 130) and 27.2% (n = 81) of the patients, respectively. Concerning medication, more than one fourth of the patients (25.8%; n = 77) were taking PPI, 35.6% (n = 106) were taking acetylsalicylic acid, 10.7% (n = 32) were taking nonsteroidal anti-inflammatory drugs, 21.1% (n = 63) were taking anticoagulants, and 12.4% (n = 37) were taking other antiplatelet agents. The most common presentation at admission was melena (54.4%; n = 162), followed by hematemesis (34.6%; n = 103), hematemesis and melena (7.7%; n = 23), and hematochezia (3.4%; n = 10). Syncope was reported by 11.7% of the patients (n = 35). Only 4.4% of the patients (n = 13) presented with hemodynamic instability at admission. PPI were started in all of the patients and erythromycin was started in 37.2% (n = 111) of the patients previous to EGD. A weekend presentation was verified in 18.5% of the patients (n = 55) and an out-of-hours admission was verified by 37.6% (n = 112) of the patients. The patients had a mean hemoglobin value of 9.0 ± 2.8, a mean platelet count of 248 ± 164, and a mean international normalized ratio of 1.6 ± 1.3 at admission. The detailed patient characteristics are indicated in Table 2.
Endoscopic Features
The most frequent sources of bleeding diagnosed in EGD were gastric ulcers (20.1%; n = 60), duodenal ulcers (17.8%; n = 53) and erosive gastritis (8.7%; n = 26). EGD was unrevealing in almost one fifth (19.5%; n = 58) of the patients. High-risk endoscopic stigmata (active bleeding or visible vessel) ulcers were present in 14.8% of the patients (n = 44). All of the patients received endoscopic treatment, except for 2 patients who were sent for immediate surgery after endoscopic diagnosis of a large duodenal vessel.
A full list of the endoscopic findings is presented in Table 2.
Clinical Outcomes
The median length of hospital stay was of 7 days (IQR 5-10). Admission to an intensive or intermediate care unit for close monitoring was considered by the attending physicians in 36.6% of the cases (n = 109). The remaining patients were admitted to a conventional ward, with the exception of 3.7% of the patients (n = 11) who were discharged. Blood transfusions were carried out in 73.2% of the patients (n = 218), with a median of 2 red blood cell units transfused (IQR 0-4). Endoscopic hemostatic treatment was performed in 31.2% (n = 93) of the patients; 48 patients had a hemostatic procedure for peptic ulcer bleeding, while the other 45 patients had an intervention in other contexts. Rebleeding after index endoscopy occurred in 7.7% (n = 23), and 3.4% (n = 10) of the patients needed another endoscopic treatment during hospital admission after rebleeding suspicion. Surgical management was required in only 2.3% (n = 7) of the patients. All-cause in-hospital mortality was 6.7% (n = 20) and the 30-day mortality was 6.7% (n = 20). Death occurred, on average, 10.4 ± 14.2 days after admission. Of the patients who had rebleeding, only 3 died in the following 30 days. The detailed clinical outcomes are specified in Table 2.
Risk Stratification and Timing of Endoscopy
At admission, the patients had a mean GBS of 11.5 ± 3.8, with 55% (n = 164) being stratified as higher-risk (GBS ≥12) and 45% (n = 134) being stratified as lower-risk bleeding (GBS <12). Higher-risk bleeding patients had a mean GBS of 14 ± 2 and lower-risk bleeding patients had a mean GBS of 8 ± 3. Endoscopy was performed within 24 h of presentation in 62.1% of the patients (n = 185), with a median timing of endoscopy of 18.7 h (IQR 9.4-32.1; Table ); this was not significantly different between the lower- and higher-risk bleeding groups (18.3 [IQR 8.7-38.4] vs. 19.3 [IQR 9.7-29.5] h; p = 0.74).
Comparing higher- versus lower-risk bleeding patients, there was no difference regarding gender (male: 59.8 vs. 68.7%; OR = 0.8; 95% CI 0.7-1.0; p = 0.11) or weekend admission (17.1 vs. 20.1%; OR = 0.9; 95% CI 0.7-1.2; p = 0.50). Higher-risk bleeding patients were significantly older (73.9 ± 13.5 vs. 66.2 ± 16.5 years; g = 0.52; p < 0.001) and had more high-risk endoscopic stigmata ulcers (20.1 vs. 8.2%; OR = 1.5; 95% CI 1.0-2.4; p = 0.04), more comorbidities (mean Charlson comorbidity index: 4.6 ± 2.3 vs. 3.5 ± 2.4; g = 0.47; p < 0.001), and a higher anticoagulant intake (26.8 vs. 14.2%; OR = 1.6; 95% CI 1.1-2.4; p = 0.01).
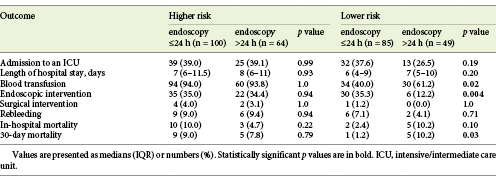
Comparing the outcome data stratified by timing of endoscopy (≤24 vs. >24 h), there were no statistically significant differences in performing endoscopy within 24 h of presentation regarding admission to an intensive/intermediate care unit (38.4 vs. 33.6%; OR = 1.1; 95% CI 0.8-1.6; p = 0.41), the median length of admission (7 [IQR 5-10] vs. 7 [IQR 6-10] days; p = 0.42), therapeutic endoscopic intervention (35.1 vs. 24.8%; OR = 1.4; 95% CI 0.9-2.0; p = 0.06), surgery intervention (2.7 vs. 1.8%; OR = 1.3; 95% CI 0.4-4.3; p = 0.71), rebleeding (8.1 vs. 7.1%; OR = 1.1; 95% CI 0.6-2.0; p = 0.75), in-hospital mortality (6.5 vs. 7.1%; OR = 0.9; 95% CI 0.5-1.6; p = 0.84), and 30-day mortality (5.4 vs. 8.8%; OR = 0.7; 95% CI 0.5-1.2; p = 0.25). Patients in whom EGD was performed within 24 h of presentation had a lower need for transfusion (69.2 vs. 79.6%; OR = 0.70; 95% CI 0.48-1.0; p = 0.048).
Higher-Risk Bleeding
We further analyzed whether the timing of endoscopy had an impact on different patient subgroups, i.e., higher- and lower-risk bleeding patients as defined in Materials and Methods. Regarding the higher-risk group, there were no statistically significant differences in outcomes in performing endoscopy within 24 h of presentation, regarding admission to an intensive/intermediate care unit (OR = 1.0; 95% CI 0.7-1.5; p = 0.99), the median length of hospital stay (p = 0.93), the need for blood transfusion (OR = 1.0; 95% CI 0.5-2.3; p = 1.0), the need for endoscopic (OR = 1.0; 95% CI 0.7-1.5; p = 0.94) or surgical intervention (OR = 1.2; 95% CI 0.4-3.7; p = 1.0), rebleeding (OR = 1.0; 95% CI 0.5-1.9; p = 0.94), or in-hospital (OR = 1.8; 95% CI 0.5-2.3; p = 0.22) and 30-day mortality (OR = 1.1; 95% CI 0.7-1.5; p = 0.79; Table 3).
Lower-Risk Bleeding
In lower-risk bleeding patients, performing endoscopy within 24 h of presentation was associated with a higher need of endoscopic treatment (OR = 2.6; 95% CI 1.2-5.7; p = 0.004), a lower 30-day mortality (OR = 0.41; 95% CI 0.27-0.63; p = 0.03), and a lower need for transfusion (OR = 0.58; 95% CI 0.36-0.92; p = 0.02). Lower-risk bleeding patients in whom EGD was performed 24 h after presentation had a 3.9-fold greater chance of dying within 30 days (OR = 3.9; 95% CI 0.66-23.7) and a 1.4-fold greater chance of needing a transfusion (OR = 1.4; 95% CI 1.0-1.8). There was a tendency toward a lower in-hospital mortality (OR = 0.49; 95% CI 0.29-0.82; p = 0.10) with performance of endoscopy within 24 h of presentation, though without statistical significance. There were no significant differences regarding admission to an intensive/intermediate care unit (OR = 1.4; 95% CI 0.8-2.4; p = 0.19), the median length of hospital stay (p = 0.20), surgical intervention (OR = 0.6; 95% CI 0.6-0.7; p = 1.0), or rebleeding (OR = 1.5; 95% CI 0.4-5.0; p = 0.71; Table 3).
Comparing the clinical characteristics of lower-risk bleeding patients regarding the timing of EGD, we verified that patients in whom EGD was performed 24 h after presentation were significantly older (70.8 ± 15.2 vs. 63.5 ± 16.7 years; g = 0.45; p = 0.01) and had a higher mean Charlson comorbidity index (4.0 ± 2.1 vs. 3.1 ± 2.6; g = 0.38; p = 0.03), a higher mean systolic blood pressure (130 ± 20 vs. 123 ± 21 mm Hg; g = 0.38; p = 0.03), a lower mean hemoglobin concentration (10.1 ± 3.1 vs. 11.3 ± 2.8 g/dL; g = 0.40; p = 0.03), more frequently a weekend NVUGIB presentation (55.1 vs. 0.0%; OR = 4.9; 95% CI 3.4-7.0; p < 0.001), and a history of heart failure (24.5 vs. 9.4%; OR = 1.7; 95% CI 1.0-2.9; p = 0.02).
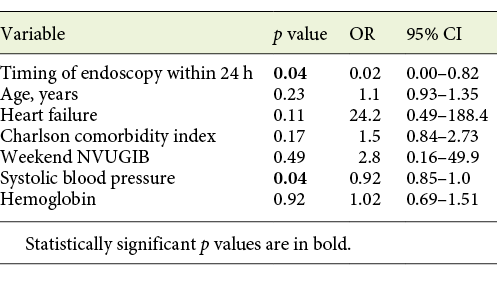
In a multivariate analysis of the possible variables associated with the 30-day mortality, including potential confounders, in the lower-risk group we found that, even after adjustment for potential confounders including age, comorbidities, weekend presentation, systolic blood pressure, and hemoglobin, performing EGD after 24 h in the lower-risk group remained a significant predictor of the 30-day mortality (OR = 0.02; 95% CI 0.0-0.82; p = 0.04; Table 4).
Table 4 Multivariate analysis of the association between timing of endoscopy and 30-day mortality in lower-risk patients, adjusting for potential confounders
In a multivariate analysis of the possible variables associated with the need for endoscopic treatment and of transfusion, in the lower-risk group we found that, even after adjustment for age, comorbidities, weekend presentation, systolic blood pressure, and hemoglobin, performing EGD after 24 h in the lower-risk group remained a significant predictor of the need for endoscopic treatment and transfusion (need for endoscopic treatment: OR = 4.7; 95% CI 1.0-22.1; p = 0.048; need for transfusion: OR = 0.78; 95% CI 0.51-0.90; p = 0.04).
Discussion
It is widely accepted that EGD should be performed within 24 h of presentation with NVUGIB [6-12]. Despite this, the optimal timing of endoscopy remains unclear. Previous studies have demonstrated that EGD within 24 h results in a reduction of the length of hospital stay and a decrease in transfusion requirements, although the impact on the need for surgery and in-hospital mortality is variable [13, 14]. In our study, patients in whom EGD was performed within 24 h of presentation had a significant lower need for transfusion and a trend towards a higher rate of therapeutic endoscopic interventions, but there were no statistically significant differences regarding admission to an intensive/intermediate care unit, length of stay, surgery intervention, rebleeding, or in-hospital and 30-day mortality.
Some guidelines suggest that, in higher-risk bleeding, EGD may be considered earlier, i.e., within 12 h of presentation [7, 9, 16, 17], based on previous studies that provided limited evidence of improved outcome in those patients when EGD was performed within 6-13 h [18, 25, 26]. However, randomized clinical trials data have suggested that EGD within 24 h is as efficacious at improving outcomes as EGD within shorter time frames (2, 6, or 12 h) [12, 24, 26-28], and more recent guidelines state that, because of conflicting data with widely heterogeneous patient populations, insufficient data exist to recommend or advise against EGD more urgently than after 24 h in higher-risk bleeding [11].
Of note, evidence that can precisely identify which patients, stratified according to bleeding risk, should undergo early endoscopy is not available and there is much controversy in this field since most of the research is characterized by low-quality observational studies including considerable selection bias, considerable confounding factors by severity of bleeding and comorbidity, and a low sample size. Moreover, patient stratification criteria were variable among studies, and features that have been considered to indicate higher-risk bleeding included GBS ≥8-12, bloody nasogastric aspirate or persistent in-hospital blood emesis, persistent hemodynamic instability (hypotension and tachycardia) despite ongoing resuscitation, contraindication for interruption of anticoagulation, and comorbidities such as cirrhosis [18-20, 24-26]. Data on the use of established risk stratification scores to select a subgroup of patients who may benefit from early endoscopy is sparse. It is known that the use of risk stratification scores to triage patients with UGIB is recommended, and several prognostic scores have been developed and compared for this purpose [29-31]; the GBS [32] is the most widely used risk assessment tool. It has been extensively validated and was developed to predict the risk of patients with UGIB of requiring a clinical intervention, including blood transfusion, endoscopy, or surgery [23, 33, 34]. In our study, higher-risk bleeding was defined by a GBS ≥12, which is in accordance with other previous studies [19, 24] and based on the work by Lim et al. [18]; they showed that a threshold value of 12 identifies patients with a higher risk of bleeding, with a specificity of 90% for predicting all-cause in-hospital mortality in NVUGIB.
The present study demonstrated that EGD performed within 24 h of presentation is associated with a significant lower need for transfusion, a lower 30-day mortality, and a nonsignificant trend toward a lower in-hospital mortality in lower-risk bleeding patients, but the same association was not demonstrated in higher-risk bleeding.
Regarding the results in lower-risk bleeding, the univariate analysis showed that patients in whom EGD 24 h after presentation were significantly older, with more comorbidities, more frequent weekend presentation, and lower hemoglobin values, which could partially explain the increase in mortality. However, the association between performing EGD after 24 h and a higher 30-day mortality was persistently significant even after adjustment for possible confounders, rendering it not negligible in this group of patients. We theorize that this delay in performing endoscopy in patients with more comorbidities was probably due to a greater difficulty in clinical management and prolonged stabilization. Recently, a large study also found that EGD within 24 h was associated with a lower in-hospital mortality compared to later endoscopy in lower-risk bleeding [20]. However, Kumar et al. [19] reported that more urgent endoscopy within 12 h, in lower-risk bleeding patients with a GBS<12 presenting with NVUGIB was a predictor of worse outcomes (a composite of inpatient death from any cause, rebleeding, need for surgical or interventional radiologic intervention, or endoscopic reintervention). Alexandrino et al. [35] also found that more urgent endoscopy within 12 h, in lower-risk bleeding, was a predictor of worse outcomes. Endoscopic therapy remains the cornerstone of therapy in NVUGIB and it is associated with improved outcomes [5]; therefore it seems plausible that the timing of endoscopy may also affect outcomes. Late performance of EGD and endoscopic therapy may increase the risk of ongoing or recurrent bleeding, thereby reducing the positive effect of the intervention. However, early endoscopy may be associated with suboptimal resuscitation and stabilization of comorbidities [19, 20]. Consequently, it is possible that there is a “window” for optimal timing of endoscopy that allows sufficient time for preendoscopic clinical optimization of the patient but does not significantly delay the performance of endoscopy [19, 35]. Based on these and our results, we suggest performing EGD in lower-risk bleeding patients with a GBS <12, between 12 and 24 h of admission, which seems to be the ideal window that allows adequate patient stabilization, though without delaying performance of endoscopy and enabling appropriate endoscopic intervention.
Concerning the results found in higher-risk bleeding, we hypothesize that mortality in the higher-risk group was mainly due to causes unrelated to UGIB, i.e., decompensation of comorbidities, since there were no differences in other clinical outcomes related to UGIB such as the need for transfusion, rebleeding, or the need for surgery. The higher-risk group had an in-hospital and 30-day mortality of 7.9 and 8.5%, respectively. Conversely, the lower-risk group had an in-hospital and 30-day mortality of 5.2 and 4.5%, respectively. As part of the management of these patients, at our institution we are aware that performing endoscopy before adequate stabilization of comorbidities may have a negative impact on patient outcomes. Therefore, we believe that the possibility of a selection bias, meaning that high-risk patients would have had an endoscopy performed earlier, was not present in our study. Moreover, we have shown that there were no differences in clinical outcomes related to UGIB, such as the need for transfusion, rebleeding, the need for surgery, in-hospital mortality, and 30-day mortality.
In our study, endoscopy was performed within 24 h only in 62.1% of the patients. We believe that EGD was not performed within 24 h of admission in a higher proportion of patients as, in our unit, endoscopy is not available 24 h/day. Thus, there is a considerable percentage of cases corresponding to weekend (18.5%) and out-of-hours bleeding (37.6%).
Studies reported an increased mortality in patients presenting to hospital on weekends for several medical conditions [36-38]. However, regarding UGIB, Jairath et al. [39] showed that there was no increase in mortality for weekend versus weekday presentation despite patients being more critically ill and having greater delays to endoscopy at weekends. In our results, weekend NVUGIB did not increase the 30-day mortality in lower-risk bleeding in multivariate analysis, ensuring that there was no confounding effect on mortality seen in those patients.
Although high-risk stigmata were found in a minority of patients (14.8%; n = 44), only 3.7% of them (n = 11) were discharged. This is explained by the fact that our population had a mean age of 70.4 ± 15.4 years, as well as several comorbidities; 27.2% had heart failure and 36.6% of the patients needed to be admitted to an intensive/intermediate care unit. In fact, it has been described that most deaths after UGIB are caused by underlying comorbidities rather than exsanguination [10], so attention to other medical problems is essential for patient management.
Our study has some limitations, such as its retrospective nature, which precluded some data collection such as smoking status or alcohol consumption, and exclusion of a considerable number of patients due to a lack of necessary records. We did not include in-hospital bleeding, i.e., patients who were admitted for reasons other than NVUGIB and subsequently developed NVUGIB during the inpatient stay, due to inaccurate records of the exact time of the onset of the bleeding event in these situations. Despite these limitations, we believe that our study is relevant because potential confounders were considered in the analysis and a nonnegligible sample of patients is presented.
In conclusion, according to our data, performance of endoscopy within 24 h of presentation, even in lower-risk bleeding patients with a GBS <12, is associated with a clinically significant decrease in the need for transfusion and 30-day mortality and a clear trend towards a lower in-hospital mortality.
Further data are needed in order to clarify the precise and optimal timing of endoscopy in patients with NVUGIB and its impact in different-risk patients, as evidence remains controversial, with conflicting data and heterogeneous patient populations.