Introduction
Objective evidence of bowel inflammation is a key feature in the management of inflammatory bowel disease (IBD) patients, since clinical-based assessment is insufficient to make adequate therapeutic decisions [1]. Endoscopic mucosal healing (MH) has emerged as a major therapeutic endpoint, as it has been associated with long-term clinical remission, steroid-free remission, and reduced risk of surgery [2,3]. However, endoscopy is a time-consuming, expensive and invasive technique, not always tolerated by patients. Therefore, a growing interest has risen regarding non-invasive monitoring tools, such as intestinal ultrasound (IUS) and faecal calprotectin (FCal). IUS is a widely available imaging modality associated with low costs, an excellent safety profile, and lack of preparation [4]. It is increasingly recognised as an accurate technique as part of the armamentarium for IBD diagnosis, but also for assessing disease activity and extent, detecting complications, and monitoring response to therapy [5]. Moreover, IUS can be performed in a point-of-care setting, leading to therapy optimisation without delay, allowing repeated evaluations to monitor lesions over time, and even replacing invasive examinations, such as endoscopy [6]. Moreover, due to lack of radiation, good availability, and because it is an easy exam to perform for both patients and physicians, in experienced hands IUS can also replace other cross-sectional image modalities, such as computerised tomography (CT) or magnetic resonance (MR) [3]. When compared to other non-invasive monitoring tools such as FCal or C-reactive protein, IUS offers additional information, namely on disease extension, location, severity, and complications [4,6]. Finally, in an era of shared decision-making with our patients, it is important to consider their acceptance when proposing follow-up examinations. In a recent systematic review, IBD patients preferred non-invasive techniques, particularly IUS, to monitor disease activity, when compared to endoscopy [7].
There is a clear need to consider IUS as a non-invasive monitoring tool in IBD, with recent ECCO-ESGAR recommendations supporting the use of IUS in the diagnosis and management of IBD patients [3]. In this review, we comprehensively discuss the role of IUS for: (a) screening and diagnosis of IBD; (b) evaluating disease activity and postoperative recurrence in Chron’s disease (CD); (c) evaluating disease-related complications; and (d) monitoring response to therapy, both in CD and ulcerative colitis (UC) patients.
Screening and Diagnosis of IBD
IUS has been used as a screening tool in patients with gastrointestinal (GI) symptoms but without severe signs of disease (such as weight loss, anaemia, or elevated FCal), showing a good accuracy to distinguish IBD from irritable bowel syndrome patients in primary care settings [8]. Additionally, in a recent prospective study including 37 patients with low-risk abdominal symptoms, the use of IUS reduced the number of colonoscopies and appointments, improving health service outcomes [9]. Furthermore, GI infections can also mimic IBD. IUS has been shown to be an accurate method in the diagnosis of infectious enteritis when compared to CT or MR, and the major findings include hypoechoic small bowel wall thickening and lymph node enlargement. Similarly, IUS can also detect inflammation in infectious colitis. Importantly, all these IUS features may overlap with IBD, and IUS alone cannot diagnose GI infections. Therefore, an ultrasound control can be performed in these patients to exclude IBD [10].
The most frequent IUS parameter used to detect intestinal inflammation is bowel wall thickness (BWT; Fig.1a). Common cut-off values are 2-3 mm for the small bowel and 3-4 mm for the colon [11]. Loss of bowel wall stratification (BWS) and increased vascularisation assessed through colour Doppler flow (CDF) are also associated with active inflammation (Fig.1b, c) [11]. Finally, extramural features are also important, such as mesenteric fat proliferation and lymph nodes (Fig.1d).
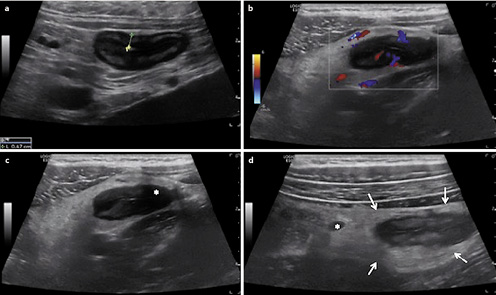
Fig.1 Examples of IUS parameters.aMeasurement of increased BWT (4.7 mm). bIncreased CDF (Limberg score 4). cAreas of focal loss of BWS (asterisk). dExtramural findings, including mesenteric fat proliferation (arrows) and mesenteric lymph node (asterisk).
Therefore, IUS can be a very helpful tool for IBD diagnosis. For instance, CD patients at diagnosis should undergo small bowel assessment, either by MR enterography (MRE), IUS, and/or capsule endoscopy. CT enterography is another valid option, though it is associated with radiation exposure [3]. In a systematic review, including 1,558 CD patients, endoscopic, histologic, barium examination, and/or intraoperative findings were used for the reference standard. The overall polled sensitivity of IUS was 88% and specificity 97% [12]. When specifically evaluating small bowel disease, the overall sensitivity of IUS ranged from 54 to 93%, with a specificity of 97-100% [13].
Several studies have assessed the value of BWT to support the diagnosis of UC [4]. Even though UC is a mucosal disease, a BWT >4 mm had a sensitivity of 62-89% and specificity of 77-88% for its diagnosis [4]. Nevertheless, the best cut-off at diagnosis is not established and values >3 mm have also been reported.
In patients with active IBD, UC patients have a prominent thickening of the mucosal layer, whereas CD patients have a significant thickening of the submucosal layer and a higher rate of lymph node enlargement [14]. In UC, the thickening of the bowel wall is mostly proportional and BWS is usually present [11]. The mesenteric proliferation is a prominent feature in CD, although it can also occur in UC, especially during severe episodes [11]. Hence, IUS is an accurate method to screen for intestinal inflammation and to support the diagnosis of both CD and UC.
Evaluating Disease Activity in IBD
Disease Activity and Postoperative Recurrence in Crohn’s Disease
IUS has shown a good accuracy in detecting disease activity in CD. In a systematic review, the overall sensitivity of IUS for assessing CD activity when compared to ileo-colonoscopy, barium-contrasted exams, CT, MRE, capsule endoscopy, or surgical specimens was 89%, with a specificity of 94.3% [5], as previously reported [15]. When compared to MRE, IUS has an accuracy of 91% for localisation and 89% for bowel wall flow [16]. Similarly, in a recent prospective study, the accuracy of IUS was not significantly different from MRE, regarding BWT, loss of BWS and CDF, also highlighting the concordance between IUS and other cross-sectional exams [17]. The METRIC trial was a prospective multicentre trial including 284 patients (133 newly diagnosed; 151 relapsed) to evaluate MRE and IUS performance in assessing disease extent and activity in CD. A constructed referenced standard was used to compare the two techniques. Both MRE and IUS were highly accurate for detecting small bowel disease, even though a higher sensitivity and specificity in detecting disease activity and evaluating disease extent was observed with MRE [18]. Nonetheless, an expert panel highlighted some methodological limitations of this study such as bias in the constructed reference standard model, absence of information on time between MRE and IUS, and use of high BWT cut-offs [19]. Importantly, the sensitivity of IUS seems to be lower for jejunal lesions (55.6%) when compared to ileal (92.7%) or colonic involvement (81.8%) [5].
Regarding postoperative recurrence, even though ileocolonoscopy remains the gold standard examination, non-invasive tools may be considered, especially after small bowel resection [20]. In a recent systematic review, the pooled IUS sensitivity and specificity for detecting postoperative recurrence was 94 and 84% [21]. Small intestine contrast ultrasonography (SICUS) had a higher sensitivity (99 vs 82%), but lower specificity (74 vs. 88%) than IUS. Also, a higher concordance between contrast-enhanced ultrasound (CEUS) and colonoscopy has been observed when compared to IUS alone (k= 0.82 vs. 0.64,p< 0.001), suggesting that both SICUS and CEUS can improve anastomosis evaluation [22]. Moreover, perianastomotic BWT correlated with Rutgeerts’ endoscopic score (r= 0.67,p= 0.0001), with higher BWT in patients with a score ≥i3 [23]. A cut-off BWT above 5.5 mm predicted severe endoscopic recurrence (≥i3) [21].
Finally, a growing interest has emerged with the use of transperineal ultrasound (TPUS) to assess perianal disease as a simple and painless method. TPUS showed a sensitivity of 90.6% and a positive predictive value (PPV) of 93.4% in detecting perianal fistulae when compared to pelvic MR [24]. Extrasphincteric and suprasphincteric fistulae were less detected by TPUS, when compared to transsphincteric and rectovaginal/anovulvar fistulae. Regarding perianal abscesses, TPUS showed a sensitivity of 50% and PPV of 79% [25]. Importantly, although not completely studied, the steep learning curve of TPUS may limit the current use of this resource in clinical practice [26]. According to previous studies, physicians may achieve competency in TPUS after 12 months of training [27].
Accompanying the increasing evidence of IUS as an accurate tool to assess disease activity, several IUS scores have been published (Table1). Six studies [28-33] evaluated inflammatory disease activity and showed a strong correlation between IUS score and endoscopy [28,29,31,32]. Additionally, an expert consensus developed the International Bowel Ultrasound Segmental Activity Score (IBUS-SAS), with an almost perfect intraclass correlation coefficient (ICC 0.97 [0.95-0.99],p< 0.001) [33]. Nevertheless, the BWT definition varied between the studies, ranging from 3 mm [28], to 4 mm [29], or even 5 mm [31] in the colon. Additionally, two studies evaluated postoperative recurrence [22,34], two compared stricture detection and echo pattern between IUS and MRE or histology [35,36], and one investigated the concordance between IUS and MRE scores based on the Lémann index (LI) [37]. Interestingly, a high concordance was found between US-LI and MR-LI (r= 0.90, p< 0.001), suggesting that IUS was not inferior to MRE to evaluate bowel damage.
Therefore, IUS is an accurate method to assess disease activity, even though a lower sensitivity when evaluating the jejunum has been shown. Regarding the postoperative setting, IUS is a useful method in detecting and grading the severity of recurrence in CD. Nevertheless, for patients with BWT <5.5 mm, IUS alone may not be sufficient to guide their management, as an accurate distinction between cicatrisation and mild to moderate recurrence may not be achieved and, therefore, cannot replace endoscopy yet [38]. Finally, several endoscopic scores have been developed but none is fully validated. Accordingly, no specific IUS score is currently recommended to evaluate CD.
Disease Activity in UC
Although the role of IUS is less well established in UC, its value in evaluating disease activity has also been explored. In a prospective study, 53 UC patients underwent colonoscopy and IUS within 1 week. Patients with endoscopic active disease had higher BWT, presence of CDF, loss of BWS, and enlarged lymph nodes [39]. In a recent systematic review, most studies showed an association between IUS findings, either defined by BWT alone or in combination with other features, and disease severity on endoscopy [4]. Moreover, the accuracy of IUS to evaluate disease extension compared to endoscopy was reported as 88.5-95% (sensitivity 95%; specificity 96%) [4]. Assessments of the sigmoid and descending colon had the higher accuracy [40], in contrast to the rectum, where transabdominal IUS had a poor sensitivity (15%) [41]. Nevertheless, this limitation could be exceeded using TPUS. In a cross-sectional study, 57 UC patients underwent transabdominal and TPUS evaluation simultaneously, 7 days before or after colonoscopy. Rectal BWT (r= 0.72, p< 0.001) and CDF (r= 0.66, p< 0.001) correlated well with the Mayo endoscopic score, suggesting that TPUS can be a good tool to evaluate patients with proctitis [42].
Considering IUS scores, nine indices have been prospectively developed in UC (Table2). All studies were based on BWT and usually complemented by CDF [39,43-46] and/or BWS [39,4,44,46,48]. Most studies considered a normal BWT when below 3 mm, even though two studies considered 4 mm to define normal BWT [45,47]. Overall sensitivity of UC scores ranged from 71 to 100% and specificity from 63.8 to 100%. A strong correlation was found between IUS scores and endoscopy [43-45,47], especially in severe disease (r= 0.94, p< 0.001) [43].
Thus, IUS has shown a good performance in assessing disease activity in UC, although a lower sensitivity has been reported when evaluating the rectum, which could be exceeded using TPUS. Similar to CD, no IUS score has been formally validated.
Evaluating Disease-Related Complications
Crohn’s Disease
Several studies have assessed IUS accuracy to detect intestinal strictures, with a sensitivity ranging from 74.4 to 100% and a specificity of 63-100% [35,49-53]. Strictures have been defined by a thickening and stiffness of the bowel wall, accompanied by a proximal dilation >2.5 cm (Fig.2) [54]. In a prospective study including 249 CD patients, the concordance between MRE and IUS for stricturing disease was high when compared to intraoperative findings (k= 0.86) [55]. SICUS seems to have higher sensitivity for detecting strictures when compared to IUS (89-94 vs. 74-76%) [56,57] and showed a good accuracy in detecting ileal stenosis and prestenotic dilation [58,59]. However, it is still not clear if IUS, including SICUS, can distinguish inflammatory from fibrotic stenosis. Nevertheless, assessment of the wall echo pattern at the stricture level may suggest the degree of fibrosis. Maconi et al. [35] concluded that strictures with a stratified echo pattern had a higher degree of fibrosis compared to those characterised by a hypoechoic echo pattern. Moreover, a reduced CDF has also been associated with a fibrotic phenotype [60]. Importantly, CEUS has also been reported as an adjuvant method to characterise strictures in CD. When compared to surgical specimens, the concordance between CEUS with inflammatory or fibrostenotic phenotype was good (k= 0.63), with a good correlation between sonographic and pathology scores for both inflammatory (r= 0.53,p= 0.004) and fibrotic stenosis (r= 0.50,p= 0.007) [60]. Finally, conflicting data have been published when evaluating sonoelastography as a possible method to distinguish fibrotic from inflammatory strictures in CD, and this modality requires further investigation [61].
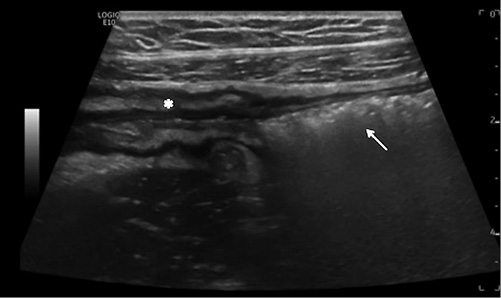
Fig. 2 IUS showing an ileal stenosis, with thickened bowel wall with narrow lumen (asterisk) and prestenotic dilation (arrow).
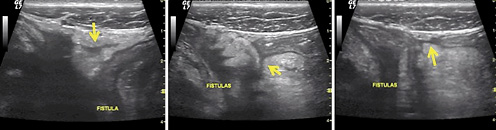
Penetrating disease is another potential complication in CD. Abscess appears as an irregular hypoechoic lesion without vascularisation. Fistulae are hypoechoic tracts, originating from the bowel wall and connecting to other tissues, such as the urinary bladder, skin, vagina, or other intestinal segments (Fig.3) [54]. In a meta-analysis, the pooled sensitivity and specificity of IUS in detecting fistulae was 74 and 95% and in diagnosing abscesses was 84 and 93%, respectively [15]. Ripollés et al. [62] showed that CEUS was able to differentiate between phlegmon and abscess in 57 CD patients, showing a high concordance (k= 0.972) with CT, MR, percutaneous drainage, or surgery. Similar findings have been previously reported, highlighting the role of CEUS as a sensitive method for differential diagnosis between phlegmon and abscess (Fig.4) [63].
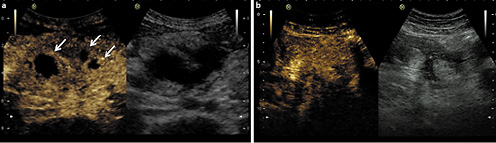
Fig.4 Two examples of CEUS showing differentiation between abscess and inflammatory mass.a Using CEUS this hypoechoic mass shows three areas completely devoid of microbubble signal, representing three abscesses. CEUS can be very helpful for defining the size of the abscesses.b Using CEUS this hypoechoic structure shows intralesional enhancement and corresponds to an inflammatory mass.
Therefore, IUS is an accurate method to evaluate CD-related complications. SICUS can help evaluating patients with strictures. CEUS supports the differential diagnosis of an inflammatory mass and is a promising tool in differentiating inflammatory from fibrotic strictures.
Ulcerative Colitis
A particular important scenario is acute severe UC (ASUC), treated with high-dose systemic corticosteroids, which is associated with an increased risk of colectomy. Nowadays, therapy response is based on clinical symptoms and biochemical markers (Oxford criteria) [64]. In hospitalised patients with moderate to severe UC, a significant decrease in BWT was observed in all patients who did not require colectomy, whereas patients who underwent colectomy had no BWT improvement between admission until day 10 [65]. In a recent pilot study including 10 patients, higher BWT (6.2 vs. 4.6 mm, p= 0.009) and any colonic segment with BWT >6 mm at admission were also associated with the need for infliximab salvage therapy. Additionally, after 3 days of high-dose steroid therapy, steroid-responsive patients had lower BWT (4.0 vs. 6.3 mm, p= 0.009) [66]. Similarly, in a retrospective study including 69 ASUC episodes in 52 paediatric patients, salvage therapy was more frequently needed in patients with higher BWT, higher vascularisation, and loss of BWS at admission. A thickened wall (>3.4 mm) and loss of BWS were independent predictors of steroid resistance [67]. Thus, if IUS parameters prove to be independent predictors of response to systemic steroids in the ASUC setting, early IUS could enable a timelier introduction of salvage therapy.
Monitoring Response to Therapy
Crohn’s Disease
Several studies have assessed IUS as a monitoring tool in CD to evaluate the response to therapy [45,68-70]. The definition of ultrasonographic remission, or transmural healing (TH), is not yet established, although it has been defined by some authors as a complete normalisation of BWT (<3 mm) with normal CDF or a complete normalisation of all IUS parameters. Additionally, definitions for ultrasonographic response have also been proposed when a sonographic improvement occurs [71]. In a prospective study, TH was associated with higher rates of steroid-free remission, lower rates of clinical relapse, and longer intervals until hospitalisation when compared to MH, suggesting that TH may be a more accurate target than MH alone in CD [72]. In the recently published STRIDE-II update, TH is considered as a potential therapeutic target but not a formal one yet [1].
The TRUST study was a 12-month prospective study to assess the value of IUS in monitoring CD, including 243 patients with at least moderately active CD (Harvey Bradshaw index >7). A significant proportion of patients had an improvement in BWT, CDF, BWS, and mesenteric fat proliferation at the end of follow-up. These ultrasonographic changes were accompanied by clinical and biochemical improvement [70]. Similarly, in a multicentre prospective study, improvement of BWT and CDF were observed after 12 weeks, increasing even more after 12 months of therapy, highlighting that IUS response at week 12 was associated with maintenance of the IUS response at week 52 [68]. Importantly, patients without IUS improvement after 1 year of therapy had a higher need for therapy intensification or surgery (65 vs. 11%, p< 0.001) [68]. Likewise, in an interim analysis of the STARDUST trial IUS sub-study including 88 CD patients, IUS response and remission after ustekinumab induction were assessed. IUS response was defined by a BWT reduction of 25% from baseline and IUS remission by normalisation of BWT, CDF, BWS, and inflammatory mesenteric fat. At week 16, IUS response and remission rates were 33.8 and 11.3%, respectively. BWT improvement was observed as early as week 4, suggesting that IUS could be a useful method to detect early response to treatment [73]. A consistent decrease in BWT was observed up to week 48. Furthermore, the overall IUS response progressively increased over time (week 48 46.3%), accompanied by a higher rate of TH (week 48 24.1%). Interestingly, normalisation of BWT was more frequent when the colon was affected compared to the ileum (50 vs. 15.8% at week 48), reflecting a faster cicatrisation of the colon [74]. A recent multicentre prospective study, including 181 CD patients treated with different types of biologic therapies, assessed IUS improvement (decrease ≥1 mm or normalisation of BWT, decrease in length of disease, Limberg score improvement, and no worsening of other IUS parameters) and TH (normalisation of all parameters) during 12 months of follow-up. After 3 and 12 months, 36.7 and 36% of the patients showed IUS improvement, with 16.4 and 27.6% achieving TH, respectively. Patients in clinical and biochemical remission had higher rates of TH. Predictive factors of TH included colonic location (aOR 3.18, 95% CI 1.11-9.10), whereas greater BWT at baseline was associated with lower rates of TH at 3 (aOR 0.70, 95% CI 0.5-0.97) and 12 months (aOR 0.58, 95% CI 0.38-0.89) [75]. Similarly, in a recent prospective study, baseline BWT and CDF, presence of disease-related complications, FCal (>250 μg/g), and male gender were associated with a higher need for steroids, optimisation therapy, hospitalisation, or surgery after 12-months of follow-up [31]. Thus, IUS features at baseline and IUS improvement during follow-up seem to be associated with disease-related outcomes. In a prospective study, including 80 consecutive CD patients, baseline and follow-up SICUS were performed (after a median of 18 months). Patients with IUS response (improvement or normalisation of BWT, decreased length of disease, without complications) had lower need for steroids, hospitalisation, and/or surgeries at 1 and 5 years of follow-up [76]. Regarding CEUS, differences in kinetic parameters derived from time intensity curves, such as peak enhancement, wash-in perfusion index, wash-in and wash-out rate, significantly improved in patients with clinical or endoscopic response, after 6 weeks of therapy [77]. Similarly, in a prospective study of IBD patients treated with vedolizumab, amplitude-derived CEUS parameters of mural microvascularisation also decreased in clinical responders after 14 weeks of therapy [78]. Altogether, these data emphasise the role of IUS as a method for monitoring the response to treatment in CD patients (Table 3).
Ulcerative Colitis
In the TRUST&UC prospective study, IUS findings in UC patients after initiating therapy for clinical relapse were evaluated during a 12-week period [79]. Overall, 178 patients with left-sided or pancolitis completed follow-up at week 12. Patients with normalisation of BWT in the sigmoid or descending colon had higher rates of clinical response. Moreover, clinical responders showed a significant reduction in BWT and CDF at week 12. These changes could be observed as early as after 2 weeks of therapy [79]. Finally, other IUS parameters, such as mesenteric fat proliferation, BWS, haustration, and ascites also improved after 12 weeks. Clinical symptoms accompanied IUS improvement, with a lower Simple Clinical Colitis Activity Index (SCCAI) at week 12 (9 vs. 2 points,p< 0.001). Similarly, a higher proportion of patients with BWT normalisation at week 12 had normal FCal values (<250 μg/g; sigmoid colon: 48.9 vs. 22.2%, p= 0.02; descending colon: 50 vs. 25%, p= 0.03) [79]. Parente et al. [45] also evaluated moderate to severe UC patients during a 15-month follow-up period. Patients who had severe IUS activity in the third month after corticosteroids therapy had a higher risk of severe endoscopic activity at 15 months (OR 9.1, 95% CI 2.5-33.5; Table 3).
Even though studies with longer follow-up are needed, these data support the use of IUS as a non-invasive monitoring tool to assess therapy response in UC. Importantly, the IUS response can be observed as early as 2-4 weeks after treatment initiation.
Future Directions and Conclusions
Nowadays, IUS is a very useful tool in the management of IBD patients, with a good accuracy in detecting disease activity, extent, and complications in CD. Besides, although being a mucosal disease, recent published data also endorse its use in UC to assess disease activity and extension. Emerging data have supported the use of IUS as a promising tool to assess response to treatment in both UC and CD, reporting changes in IUS features as early as 2-4 weeks of treatment and that persist in short- and long-term follow-up (Fig.5). In fact, this could lead to a paradigm change in IBD, as IUS can become a routinely used tool in the management of these patients in a point-of-care setting and enabling early intervention if needed. Nevertheless, the use of IUS is not yet universal and its performance is highly dependent in the operator’s experience. Inter-observer agreement of IUS in UC and CD patients is excellent for BWT and good for CDF, with fair to moderate agreement in other IUS parameters, such as lymph nodes and inflammatory fat [80,81]. Moreover, IUS can have lower accuracy in specific bowel locations, such as the proximal jejunum and rectum. Other possible limitations of IUS include the patient’s biotype, as evaluation in obese patients is difficult [82], evaluation of disease activity/extent in the postoperative setting, due to anatomical changes, and the lower capacity to detect superficial lesions in the small bowel. Therefore, it is important to train IBD-specialised gastroenterologists in this technique, as proposed by the International Bowel Ultrasound (IBUS) group. Additionally, future studies are needed to improve IUS capacity in differentiating the severity of endoscopic recurrence in the postoperative setting, as well as to deepen the knowledge on elastography and better characterisation of stricture subtype in CD. Regarding UC, the real accuracy of IUS to predict histologic remission has never been formally studied. In an era of strict endpoints like endoscopic Mayo score of zero or even histological remission, IUS parameters might not be sensitive enough to capture subtle inflammatory mucosal changes. Finally, no IUS score has been fully validated and a homogenous approach of IUS parameters is warranted to spread its use in IBD clinics and hospitals, as well as in clinical trials.

Fig.5 The current role and future directions of IUS in IBD. ASUC, acute severe ulcerative colitis; CD, Crohn’s disease; GI, gastrointestinal; IBS, irritable bowel syndrome; TPUS, transperineal ultrasound; UC, ulcerative colitis.
In conclusion, IUS is an accurate non-invasive monitoring tool not only to assess IBD diagnosis, disease extent, and activity in CD and UC, but also to monitor response to therapy. In experienced hands, IUS adds extraordinary value to the management of IBD patients.