Introduction
In its decompensated state or with portal hypertension, liver cirrhosis is associated with significant changes in the human immune system leading to a syndrome called cirrhosis-associated immune dysfunction [1]. This dysfunction, related to the increase in intestinal permeability, results in bacterial and endotoxin translocation to mesenteric lymph nodes and other external sites, causing infectious complications [2]. Spontaneous bacterial peritonitis (SBP) is one of the most frequent complications. The diagnosis of SBP is established when the polymorphonuclear (PMN) leukocyte count in the ascitic fluid (AF) is equal to or greater than 250 cells/mm, in the absence of intra-abdominal cause for infection (i.e., surgically treatable) [3, 4].
SBP has an estimated prevalence of 1.5-3.5% in ambulatory patients and up to 10% in hospitalized patients [5]. A prospective study reported a rate of 47% of bacterial infections in hospitalized cirrhotic patients, with 31% of these infections being SBP [6].
SBP is associated with higher mortality rates, ranging between 18 and 33% in some series [7]. Nonetheless, an early diagnosis, related to adequate therapy, allows a decrease in disease-related mortality [7].
Guidelines recommend that a diagnostic paracentesis should be performed at admission in cirrhotic patients with: (i) ascites (who require hospitalization); (ii) local or systemic symptoms (abdominal pain, tenderness, vomiting, diarrhea, hyper/hypothermia, tachycardia and/or tachypnea) (iii) signs of clinical deterioration such as hepatic encephalopathy, gastrointestinal bleeding or worsening of renal and/or liver function [8, 9].
Adequate handling of AF is required to increase the accuracy of SBP diagnosis. The injection of 1 mL of fluid into a purple-top ethylenediaminetetraacetic acid allows a more accurate cell count; moreover, a bedside injection of at least 10 mL into aerobic and anaerobic blood culture bottles increases the accuracy of positive cultures [10].
When the culture is positive (approximately 40% of cases), the most common pathogens include Gram-negative bacteria, mainly Escherichia, with Escherichiacoli being the most prevalent [5, 10, 11]. Gram-positive cocci have previously accounted for less than 25% of SPB cases; however, there is a recent increased prevalence [12].
Antibiotic therapy should be initiated early to improve outcomes of disease [12]. Third-generation, broad-spectrum cephalosporins are the drugs of choice for community-acquired infections. Cefotaxime (2 g every 8 h) during 5 days is considered the gold-standard therapy [8, 9, 13]. Ceftriaxone (2 g/day for 5 days) is an acceptable alternative [14]. Albumin should be administered (1.5 g/kg at diagnosis and 1 g/kg on day 3) to avoid type 1 hepatorenal syndrome [may occur in approximately 30% of SBP patients (treated with antibiotics alone)] and improve survival [8].
European guidelines suggest a new paracentesis 48 h after the beginning of antibiotics to demonstrate SBP resolution (by a decrease in PMN cells >25%) and to adjust therapy if needed [8]. However, some recent studies consider that this procedure is unnecessary for all patients and could be individualized according to the clinical and analytical course [9, 15].
With this study, the authors aim: (i) to determine the patients who may benefit from follow-up paracentesis according to clinical and analytical predictors of inadequate response on day 3 of treatment, and (ii) to create a predictor model of inadequate response to antibiotic therapy.
Material and Methods
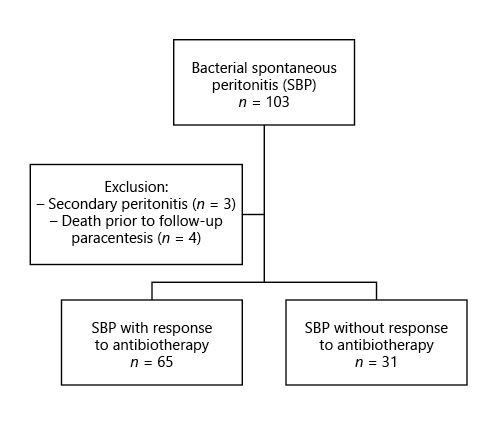
We have performed a retrospective single-center study (in a tertiary center) including all consecutive adult patients admitted with SBP between January 2011 and June 2018. Patients who died from causes other than SBP during that period were excluded (Fig. 1).
Data from serum laboratory workup at admission and 48 h after the beginning of antibiotics included: blood count with platelets, cytocholestatic parameters, serum proteinogram, urea, creatinine, and C-reactive protein levels. Moreover, an initial AF evaluation was performed, simultaneously, with a differential count of fluid cells and microbiologic culture. Information regarding clinical aspects, namely abdominal pain and fever, were also collected. In all cases, antibiotics and albumin were initiated as soon as possible. Adequate antibiotic therapy response was considered when AF neutrophil count decreased more than 25% related to pre-treatment value after 2 days of antibiotic treatment [8]. According to a Numerical Rating Scale, abdominal pain was felt when it was classified, by every patient, as 2 points [16]. Active alcohol consumption was defined as a consumption ≥20 g in women or 30 g in men. Hepatic encephalopathy was considered over 2 on the West Haven scale. Respiratory insufficiency was defined as PaO2 <60 mm Hg. Acute kidney injury was defined according to the European Association for the Study of the Liver (EASL) criteria [8].
Statistical analysis was performed using SPSS v. 26 (IBM®, Armonk, NY, USA). Data were analyzed using a χ2 test for categorical variables, independent-samples t test, and Mann-Whitney U nonparametric test for continuous variables. Multivariate analysis using binary logistic regression was used; the variables included as predictors were selected from univariate analysis if p < 0.1. Model discrimination was measured using the area under the receiver operating characteristic curve (AUROC), considering 95% confidence intervals (CIs). Statistical significance was considered if the p value was less than 0.05.
Results
General Characteristics and Demographics
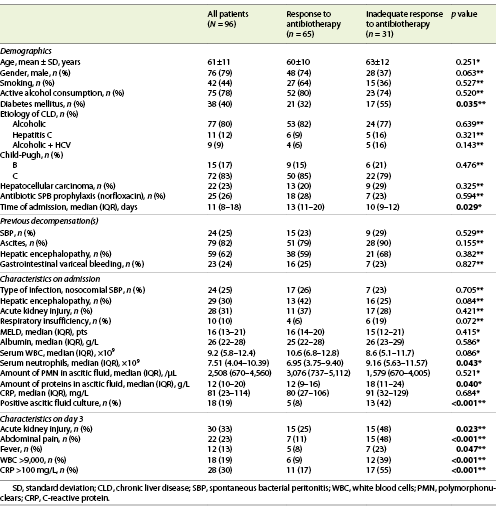
We have included 96 episodes of SBP in 75 patients (79% male sex, mean age 61 ± 11 years old). Demographic data are shown in Table 1. The median time of admission was 11 days (IQR 8-19), the period corresponding to the hospital stay. In 53% of cases (n = 51), ceftriaxone (2 g) was used as initial therapy, and in 33% (n = 32) piperacillin-tazobactam (4.5 g), with renal adjustment if necessary. In 80% (n = 77) of cases, the etiology of cirrhosis was alcoholic, and 83% (n = 72) of patients had a Child-Pugh C disease stage. Nosocomial SBP occurred in 25% (n = 24) of the cases. Thirty percent of patients were on antibiotic prophylaxis with norfloxacin (400 mg/day), and 25% (n = 24) had a previous diagnosis of SBP. Sixteen percent were on rifaximin (550 mg, twice a day) at the time of SBP diagnosis.
Outcome
An inadequate response to antibiotic therapy was observed in 30% of cases (n = 31) and, according to its response, patients were divided into 2 groups: those who responded to antibiotic therapy (group 1, n = 65) and those without an adequate response (group 2, n = 31) (Table 1).
Twenty percent of patients died during admission (6 of them due to causes unrelated to peritonitis). Thirty-nine percent of patients had DM, and this pathology was more prevalent in group 2 (p = 0.035).
Admission
As we can observe in Table 1, a higher median count of serum neutrophils [9.16 (5.63-11.57) vs. 6.95 (3.75-9.40) × 109, p = 0.043], as well as a lower median of total proteins in AF [12 (9-16) vs. 18 (11-24) g/L, p = 0.040], were related to an inadequate response to therapy (group 2). Additionally, a positive microbiologic culture of AF (p < 0.001) was more frequently found in this group.
Day 3 after Antibiotic Therapy
On the 3rd day of therapy, it was observed that patients with an inadequate antibiotic response had higher counts of serum white blood cell count (WBC) >9 × 109 (p < 0.001), C-reactive protein (CRP) >100 mg/L (p < 0.001), fever (p = 0.047) and abdominal pain (p < 0.001), than group 1.
Paired Analysis
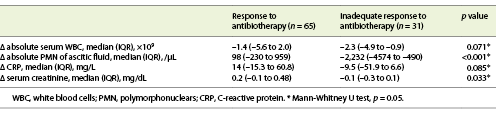
In both groups, a decrease in the serum count of leucocytes, creatinine, CRP and PMN of AF was observed. However, this decrease was more significant in patients of group 1 for PMN of AF count and serum creatinine (p < 0.001 and p = 0.033, respectively) (Table 2).
Predictors of Antibiotic Failure
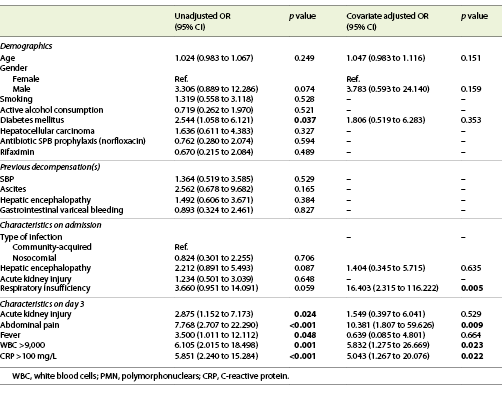
In multivariate analysis, the presence of respiratory insufficiency (OR = 16.403; 95% CI: 2.315-116.222; p = 0.005) and abdominal pain (OR = 10.381; 95% CI: 1.807-59.626; p = 0.009) at admission, serum WBC ≥9 × 109 (OR = 5.832; 95% CI: 1.275-26.669, p = 0.023) and CRP >100 mg/L (OR = 5.043; 95% CI: 1.267-20.076; p = 0.022) at day 3rd of antibiotic therapy were predictors of an inadequate response to antibiotics in SBP (Table 3).
A predictor model of inadequate response to therapy was created [-3.816 + (2.797 × respiratory insufficiency) + (2.340 × abdominal pain) + (1.763 × serum WBC >9 × 109) + (1.618 × CRP > 100)] with a good accuracy (AUROC 0.893, p < 0.001); for a cut-off of 0.090, this model has a sensitivity of 97% and a specificity of 46%, with a positive predictive value of 83% and a negative predictive value of 77% (Fig. 2).
Discussion
EASL guidelines recommend an assessment of AF through a paracentesis with cytological analysis of fluid at day 3 of antibiotics for all cases of SBP to monitor response and adjust therapy, if needed [8]. In fact, in clinical practice, an intermediate analysis of AF may play an important role, namely in matters of unfavorable clinical course. It allows therapy orientation in cases of non-response and can be a clue for a specific cause for the increased count of PMN.
However, our work meets other recent studies that suggest an individualized approach when following SBP cases [9]. Moreover, this is one of the first studies in the literature that link demographic and clinical factors to the convenience (or not) of an intermediate evaluation of AF by paracentesis. By evaluating clinical and analytical parameters in patients with SBP, we could depict some factors, both at admission and on day 3 after therapy, that can predict an inadequate response to antibiotics. Furthermore, as shown in Table 2, the behavior of clinical and analytical variables between admission and patient’s reevaluation corroborates this hypothesis of prediction of antibiotic therapy response.
Using previously reported variables, the authors created, for the first time in the literature, a predictive model to determine which patients can benefit from a second paracentesis to adjust antibiotic therapy (in case of inappropriate response) and to avoid dispensable invasive procedures (in patients with favorable outcomes). Therefore, our model allowed the identification, with reasonable accuracy, of individuals in whom the following paracentesis may have benefits, with a sensitivity of 97% and a negative predictive value of 77%. Furthermore, if we applied this model in our population, 65% of the patients could have avoided the follow-up paracentesis, thus avoiding further complications in frail patients.
As a complication of cirrhosis, SBP tends to indicate a significant progression of the disease. This is the reason why the identification of risk factors for SBP has an essential role in evaluating the disease course [17]. In our cohort, DM was present in 39% of patients, with a trend more prevalent in group 2. Tergast et al. [1], in their study, determined DM as a risk factor for SBP development in patients with cirrhosis due to alterations of the immune system, namely in leukocyte function, and to the polyneuropathy induced by DM. These changes lead to dyskinesia of bowel muscles and prolonged intestinal transit time, with an increased risk for bacterial translocation from the gut [18].
An interesting finding was the higher prevalence of positive AF cultures at admission in patients who did not respond to antibiotics. This fact emphasizes the role of a fine collection of AF sent to microbiologic analysis. According to the American Association for the Study of Liver Diseases (AASLD), in cases of SBP suspicion, AF should be cultured, at the bedside, in aerobic and anaerobic blood cultures bottles, prior to initiating antibiotic therapy [9]. Some studies report a higher rate of bacterial growth when AF is collected in this manner when compared to other containers (80 vs. 50%) [15]. Therefore, a positive AF culture is an essential guide of antibiotic therapy, mainly in patients with inadequate response to empirical treatment. Moreover, we have previously reported a higher prevalence of multidrug-resistant (MDR) bacterial infections in our center, namely SBP. We believe that SBP prophylaxis with quinolones is associated with the emergence of MDR infections [17]. In our study, we only obtained a positive culture in 48% of cases, and this could be related to an inappropriate collection of AF at the diagnostic paracentesis. This may be considered a potential limitation of our study and could be explained by the fact that initial paracentesis was performed in the emergency department (with some limitations in AF collection). Moreover, as a retrospective study, some information about the AF collection was unclear, namely the type of culture bottle. Some other limitations of the study should be acknowledged, such as selection bias may not be avoided entirely. Therefore, prospective and multicenter studies are needed to confirm these results.
The vast majority of paracenteses occurred without complications. However, some studies reported a 10% rate of adverse events, commonly minor complications, such as the continuous outflow of AF from the puncture site or local self-limited bleeding. Nonetheless, significant events can occur, like abdominal hematoma, bleeding into the peritoneal cavity or secondary peritonitis related to visceral perforation [9, 18]. In our study, two cases of continuous outflow of AF from the puncture site and one case of abdominal hematoma were seen.
With this study, the authors consider that clinical and analytic factors allow predicting the (un)response of antibiotic therapy. The following paracentesis should be reserved to patients with a predictable absence of response to adjust treatment. We are convinced that our results may have important implications for clinical practice and future studies.