Introduction
Gastrointestinal subepithelial tumors (GI-SETs) include a wide range of submucosal lesions whose prognosis may vary from benign and indolent to malignant and potentially aggressive neoplasia, such as neuroendocrine (NET) and gastrointestinal stromal tumors (GIST) [1].
Usually asymptomatic, most GI-SETs are diagnosed as incidental findings during screening endoscopy or radiological examinations. Some studies revealed that less than 10% of these lesions exhibit a significant increase in size at follow-up [2]. Despite a wide range of different histopathologic lesions, endoscopic aspect of GI-SETs is similar as they appear like smooth bulges of the inner cavity of GI tract with normal or ulcerated overlying mucosa. For this reason, when a GI-SET is suspected, endoscopic ultrasonography (EUS) examination should be performed to rule out extraluminal compression and delineate the most likely histological layer of tumor origin [3-5]. Although histology is needed for a definite diagnosis, several sonographic features, such as size, borders, echogenic homogeneity, vascularization, presence of anechoic areas, or lymph node metastases may be helpful to predict the nature of the submucosal tumor [3, 6, 7]. The management of smaller, asymptomatic GI-SETs with malignant potential or large benign lesions presenting with GI bleeding includes endoscopic resection as alternative to surgical intervention [8, 9]. In this study, we report the efficacy and 1-year outcome of endoscopic submucosal dissection (ESD) for GI-SET treatment.
Patients and Methods
Patients
Data of patients with endoscopically treated upper GI-SETs in 4 third-level endoscopy centers (Modena, Napoli, Milano, Perugia) between July 2014 and January 2020 were retrospectively reviewed. All patients included underwent standard gastroscopy, and by bite-on-bite, biopsies were obtained on lesions. When histological diagnosis was inconclusive, both radial and linear EUS were performed for adequate endosonographic evaluation, and EUS-guided fine needle biopsy sampling was carried out. Before endoscopic resection, all patients underwent CT scan to exclude local infiltration or lymph node metastasis when a malignant lesion was detected. Endoscopic resection was proposed for bleeding or symptomatic benign lesions (leiomyoma and lipoma), as well as for superficial low-risk GIST exhibiting very narrow connection with the muscular layer (type I and II) and non-ampullary NET with diameter less than 10 mm [10]. Informed consent was obtained before procedure in all patients. Since no experimental drugs were administered, no additional costs or procedures for the patients were required, no identification of patients was allowed, and no funds were received; the Investigational Review Boards waived formal approval, deeming the study to be an extension of existing clinical practice. Patients were informed and signed their consent for the procedure and the anonymous use of their data for scientific purposes.
Endoscopic Procedures
All ESD procedures were performed in general anesthesia by skilled operators in submucosal dissection with at least 10 years of practice in therapeutic endoscopy and experience of ESD training in Japan. A standard single-channel gastroscope with a water-jet system (GIF-H190; Olympus, Tokyo, Japan) was used, and transparent hood (ND-201-11802; Olympus) was applied to the distal tip of the endoscope. A high-frequency generator (VIO300D; ERBE, Tübingen, Germany) was used during mucosal incision and submucosal dissection. For mucosal incision, Endocut I mode (Effect 2) was set, while submucosal dissection was performed using Swift Coag mode (Effect 3, 40W). Carbon dioxide insufflation was used during all ESD procedures. ESD was performed after initial injection of solution (100 mL saline solution, 5 mL 0.8% indigo carmine, and 1 mL epinephrine) with a 23-gauge disposable needle into the submucosa and circumferential mucosal incision, at 1 cm from the mucosal bulge, was performed with Dual Knife (KD-650L, Olympus) or Dual Knife J (KD-655L, Olympus). Then, sub-mucosal dissection was continued close to the muscular layer and below the subepithelial lesion. When the tumor originated from the muscularis propria, submucosal dissection was completed with IT-Knife 2 (KD-611L) or Hook Knife (KD-620RL, Olympus) to grasp and remove the muscularis propria fibers along the capsule of the tumor. Major blood vessels as well as any intraprocedural bleeding were managed with Coagrasper (FD-410LR, Olympus). A careful inspection of the resection site at the end of the procedure was performed to coagulate exposed blood vessel or identify and treat any microperforation with through-the-scope (TTS) endoclips. En bloc resection was defined as excision of the tumor in only one piece with no evidence of macroscopic tissue remnant. Post-ESD complications requiring therapeutic intervention, such as perforation or bleeding, were defined as early or late events accord-ing to the time of onset, namely, within or after 48 h following the endoscopic procedure, respectively. Post-ESD cutting sites were treated in all cases by TTS positioning as the first attempt, in order to prevent and reduce the risk of bleeding and late perforation. Following endoscopic procedure, proton pump inhibitor therapy was administered to all patients, intravenously for 5 days and then switched to oral for 4 weeks at discharge. Broad spectrum antibiotics were administered to all patients for 7 days. Oral feeding was reintroduced 48 h later if the patient was asymptomatic and no bleeding was suspected. Endoscopic control for local recurrence was scheduled 3 and 6 months after endoscopic resection and then yearly in malignant lesions.
Histological Examination
Removed lesions were fixed by using 10% formalin solution, embedded with paraffin, and sectioned for histological evaluation at 2 mm intervals. Experienced GI pathologists assessed the histological type, macroscopic appearance, tumor size, depth of invasion, lymphatic and vascular involvement, capsule integrity, and resection margins. R0 resection was defined as en bloc resection with intact capsule and/or at least 2-mm free margins were present at histology. Immunohistochemistry was performed on 3 microns of thickness section for NET, GIST, and mesenchymal tumors with uncertain histopathological diagnosis. In detail, chromogranin-A and synaptophysin stains were used to confirm the diagnosis of NETs, while Ki-67 and the mitotic index were applied to define the tumor’s differentiation degree. Histological diagnosis of GIST included C-Kit, DOG1, and CD34 immunostains. Other immuno-histochemical markers were used for the diagnosis of stromal tumors and included S100, smooth muscle actin, and desmin. The lesions removed from the duodenum and histologically defined as Brunner’s hamartomas when proliferation of Brunner’s glands, organized in lobules and with marked cystic dilatation lined by columnar cells, were detected. Glands were intermingled to stromal cells and vascular spaces without atypia. The lesion probably originated from the subepithelium but deepened to the submucosa layer, however, without having invasive characteristics. Preprocedure histopathological diagnosis was achieved in 63 (75%) out of 84 patients, and it was eventually confirmed in all these cases on the resected specimen.
Results
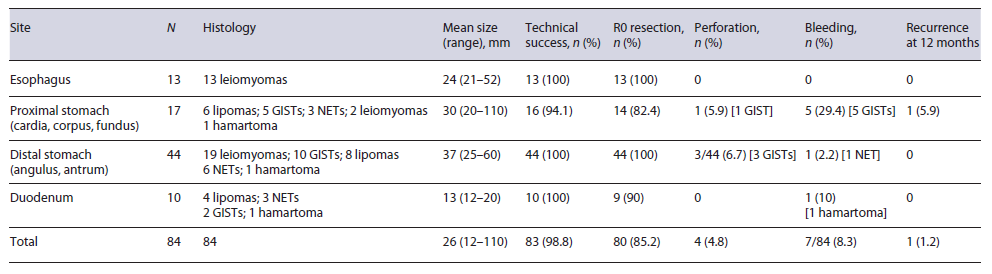
A total of 84 patients (56 males; mean age 63.5 years, range: 33-89) with upper GI-SETs were endoscopically treated, including 13 localized in esophagus (distal tract), 17 in proximal stomach (corpus/fundus/cardia), 44 distal stomach (antrum/angulus), and 10 in the duodenum (8 in the bulb and 2 in the second portion).
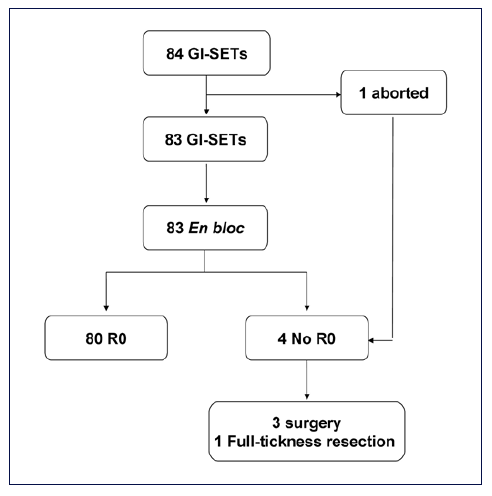
The mean diameter of the resected lesions was 26 mm, ranging from 12 to 110 mm. The mean ESD procedural time was 53 min (range: 30-160). The procedure was successful in all but 1 patient, in whom it was aborted for technical difficulty. En bloc and R0 resection were achieved in 83 (98.8%; 95% CI = 96.5-100) and in 80 (95.2%; 95% CI = 90.1-99.8) patients, respectively. At histological assessment, there were 17 GISTs, 12 NETs, 34 leiomyomas, 18 lipomas, and 3 hamartomas. In the 4 patients in whom R0 was not achieved, surgical laparoscopic-assisted gastric wedge resection was performed in 3 cases (1 gastric large, bleeding leiomyoma; 2 gastric NETs) and endoscopic full-thickness resection (EFTR) in the remaining patient (duodenal bulb NET) by using full-thickness resection device (FTRD® - Ovesco Endoscopy, Tubingen, Germany). A complete lesion removal was his-tologically confirmed in all these cases (Fig. 1).
Overall, a complication occurred in 11 (13.1%; 95% CI = 5.9-20.3) patients. In detail, major bleeding was observed in 7 (8.3%) patients, including 5 with gastric GISTs (3 fundus, 2 corpus), one with NET, and one with hamartoma of the duodenal bulb. Endoscopic hemostasis with adrenaline and TTS clips was successfully obtained in 4 out of 7 patients, while an 11-mm atraumatic with 6-mm cap (11/6) OTSC was necessary to control the bleeding in 2 patients, and radiologic embolization in a patient with a duodenal hamartoma. In this case and in a patient with a GIST of the gastric fundus, gastrointestinal hemorrhage presented with acute severe anemia and clinical feature of hypovolemic shock within 24 h after the procedure, while in the other 5 cases, the bleeding occurred during the endoscopic resection. A perforation occurred in 4 (3.6%) patients. In detail, the complications were immediately observed after removal of antral GIST in 3 cases, two successfully treated at endoscopy with 11/6 traumatic OTSC positioning, while the other patient underwent surgical intervention with subtotal gastrectomy. In the remaining case, a late perforation occurred on third day postresection of a gastric fundus GIST, and the patient was treated with surgical intervention of total gastrectomy. Overall, a surgical approach was eventually needed in 5 (5.9%; 95 CI = 0.9-11), including 3 in whom R0 resection failed and 2 with perforation. The mean hospital stay was 5.1 ± 1.3 days.
At 3 and 6 months of follow-up, no local recurrence was described, while at 12 months follow-up, relapse of 10-mm subepithelial tumor was observed in only 1 patient after resection of an ulcerated large lipoma of the gastric corpus so that a full-thickness resection was performed. No fatal events were registered at follow-up. All data were summarized in Table 1.
Discussion
ESD is a minimally invasive technique allowing to remove large GI lesions with low risk of recurrence, without resorting to a more invasive surgical approach [2, 9]. Notably, this procedure might be particularly useful for endoscopic treatment of symptomatic (bleeding, obstructive) GI-SETs, including benign masses, as well as GISTs or NETs within specific size limits and without suspicion of locoregional involvement. Indeed, these lesions generally exhibit a low malignant potential so that surgical treatment with lymph node dissection is not mandatory [10-12]. In detail, this endoscopic approach could be paricularly useful for duodenal and cardia SETs, representing a valid alternative to demolitive surgery associated with a higher rate of morbidity and mortality [13, 14]. However, ESD is challenging when the lesion is localized in some GI sites, such as the duodenum or fundus, where the risk of complications increases even when performed by skilled operators [15, 16]. In addition, dissection may result particularly difficult for lesions originating from the muscle layer or when they are larger than 5 cm, increasing the risk of perforation up to 20% [17].
ESD feasibility for GIST treatment should be evaluated according to their location in the gastric wall and their connection with the muscularis propria [18]. Indeed, ESD appears to be a good option for lesions protruding into the luminal gastric side with very narrow contact with the muscle layer (type I) or for GI-SETs, still protruding into the stomach with an obtuse angle, showing a wider contact with muscle fibers (type II). On the contrary, GISTs located in the middle of the gastric wall (type III) or exhibiting extraluminal growth (type IV) should be evaluated for surgical intervention, EFTR technique or combination of both [10]. Indeed, the improvement of EFTR has provided a less invasive treatment alternative to surgery, allowing a deeper resection, compared to ESD, of large size submucosal tumors or lesions involving the muscularis propria [19].
Largely performed in Asian centers, data on ESD removal in western countries are still scanty. Data of our case series, including different GI-SETs, showed that ESD is a successful approach, with very high values of both en bloc and R0 resection rates. In detail, a complete lesion removal, with histological free margin resection, was achieved in more than 95% of cases, which is a value in agreement with the results reported in Asian series. Nevertheless, data of some studies found a lower (72%) R0 rate when ESD was performed for GIST or other lesions arising from the muscle layer, most likely due to a strict connection between the tumor and the muscularis propria that increases the difficulty of the procedure and the risk of complications [11, 15]. Of note, in our series, all the 4 perforations occurred in removing gastric GISTs as well as 5 (75%) out of the 7 major bleedings occurred after resection of this type of lesions. This result may depend on the huge vascularity typical of this subgroup of tumors and their origin from the muscle layer. Based on these observations, we would suggest paying particular attention during GIST removal. Overall, the rate of complications is acceptably low, and both bleeding and perforation were generally suitable for an endoscopic approach. In-deed, the surgical intervention was eventually needed in only 6% of cases, due to either complication or incomplete lesion removal, in agreement with data from previous studies [9, 14].
In our study, GI-SETs showing invasion deeper than muscularis propria at EUS were excluded because large repair of the gastrointestinal wall would be required after the standard procedure of ESD. However, recently, many other techniques have been described to provide a more conservative resection of submucosal lesions, including EFTR with endoscopic suturing of the wall defect, sub-mucosal tunneling endoscopic resection, or laparoscopic endoscopic cooperative surgery procedures. With the EFTR approach, an endoscopic full-layer resection including the serosa is initially performed resulting in an intentional perforation. Then, the transmural wall defect is closed by using endoscopic suturing device (Apollo En-dosurgery, Austin, TX, USA), OTSC, or combination of TTS endoclip and endoloop [20, 21]. On the other hand, the submucosal tunneling endoscopic resection procedure allows the resection of submucosal masses without transmural loss of integrity of the gastrointestinal wall. Indeed, starting a mucosal incision about 2-3 cm from the target lesion, a submucosal tunnel is created to approach the GI-SET and then dissect the tumor from the surrounding tissue and muscularis propria. Finally, laparoscopic endoscopic cooperative surgery procedures combine the technique of ESD to determine the precise cutting line around the gastric or duodenal SETs followed by laparoscopic wedge resection. Although scientific data are scanty, all these procedures have shown good results in terms of efficacy and safety, representing a valid alternative for GI-SETs requiring full-layer resection [22]. Some potential limitations of our study might be put forward. It was a retrospective evaluation of available data, and the 1-year follow-up may be inadequate to evaluate longterm outcome. Moreover, ESD was not compared to other endoscopic techniques. In conclusion, our experience demonstrated that ESD, performed by high-trained endoscopist, may be a valid and safe alternative to surgical intervention for both benign and localized malignant GI-SETs.