Introduction
Small bowel adenocarcinoma (SBA) is a rare malignancy accounting for less than 5% of gastrointestinal cancers, with an incidence rate of 0.2-0.3/100,000 person-years in the general population [1]. Although Crohn’s disease (CD) is associated with a 22-fold increased risk of SBA, this is an unusual complication that develops during the course of CD in approximately 0.2% of patients [2] at an incidence rate of 0.3/1,000 person-years [3] and usually appears at a much younger age than in the general population [4].
Diagnosis of SBA associated with CD can be quite challenging. Obstruction is the most common presenting manifestation, whereas less common clinical presentations include hemorrhage, fistula, or perforation. Unfortunately, all of these symptoms are hard to distinguish from those of a CD exacerbation. Besides, these malignancies are often radiologically indistinguishable from long-standing CD and imaging techniques may miss small lesions. The result is that the majority of cases are diagnosed at the time of operation or postoperatively at an advanced stage [4]. The prognosis of SBA in CD is usually unfavorable with a 5-year survival of 20-30% [5].
We report a case that illustrates diagnostic difficulties associated with SBA in patients with CD and that aims to increase clinicians’ awareness of this rare but severe complication and to demonstrate that balloon-assisted enteroscopy may play an important role in achieving an early diagnosis.
Case Presentation
A 48-year-old male with a previous medical history of CD presented with iron deficiency anemia. His hemoglobin level was 10.7 g/dL, with mild microcytosis (86.2 fL) and low levels of both serum iron (33 mg/dL) and transferrin saturation (9%). The patient reported melena approximately 1 month earlier but was currently asymptomatic. He denied abdominal pain, diarrhea, or weight loss. There were no other laboratory abnormalities associated with underlying disease activity, including leukocyte and platelet count and C-reactive protein levels, which were normal. Stool calprotectin level was also normal. His last ileocolonoscopy, performed 5 months earlier, was also normal with no signs of inflammatory activity along colon and terminal ileum. CD had been diagnosed 20 years earlier and was characterized by ileal involvement and stenosing behavior, with associated perianal fistulizing disease (Montreal classification: A2L1B2p). There was a history of segmental enterectomy for ileal stenosis and anal fistulectomy performed 4 and 6 years after initial diagnosis, respectively. His current medications included azathioprine and infliximab (5 mg/kg every 8 weeks), which he had started 16 and 10 years earlier, respectively. The last acute exacerbation of CD requiring induction therapy with intravenous corticosteroids had occurred 8 years before and CD appeared to be in clinical and endoscopic remission since then.
Intravenous iron replacement with weekly injections of 200 mg of iron oxide was started. However, after 8 weeks, hemoglobin level had decreased to 9.2 g/dL despite correction of iron deficiency. The patient remained asymptomatic and laboratory studies once again did not reveal leukocytosis, thrombocytosis, or elevated C-reactive protein levels. Computerized tomography (CT) enterography revealed multiple ileal strictures with wall thickening, vasa recta engorgement, and prominent mesenteric lymph nodes, suggestive of inflammatory strictures. In addition, a sacculation with circumferential thickening of proximal and distal bowel loops extending for 47 and 31 mm, respectively, was found at the region of ileo-ileal anastomosis, as shown in Figure 1. Therefore, the patient underwent double balloon-assisted retrograde enteroscopy which revealed several ileal strictures, easily traversed with the enteroscope, and an area of infiltrative appearance at the region of ileo-ileal anastomosis, with a polypoid component (as shown in Fig. 2a) and ulceration (as shown in Fig. 2b), producing a stenosis (as shown in Fig. 2c) that could not be traversed by the enteroscope. Biopsies of this area were performed. Remarkably, histopathological examination revealed tubular adenocarcinoma infiltrating the muscularis mucosae, as shown in Figure 3.
CT scan of the thorax, abdomen, and pelvis did not reveal secondary involvement of lymph nodes, lungs, liver, or other organs. The patient underwent right hemicolectomy plus segmental enterectomy of the anastomotic region where the neoplasia was located. Histopathological examination of the surgical specimen confirmed a diagnosis of mucinous SBA with infiltration of muscularis propria and lymphatic and vascular invasion (postresection stage: pT3NxM0). Immunohistochemical analysis revealed expression of MLH1, MSH2, MSH6, and PMS2. After surgery, the patient started adjuvant chemotherapy with oxaliplatin, leucovorin, and fluorouracil. After 2 months, he is asymptomatic and follow-up CT reveals no evidence of recurrence.
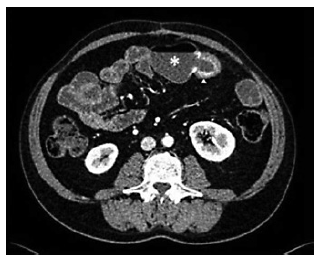
Fig. 1 Computed tomography enterography. A sacculated small bowel loop may be seen at the region of ileo-ileal anastomosis (asterisk) associated with circumferential and irregular thickening of the afferent and efferent small bowel loops (arrowhead).
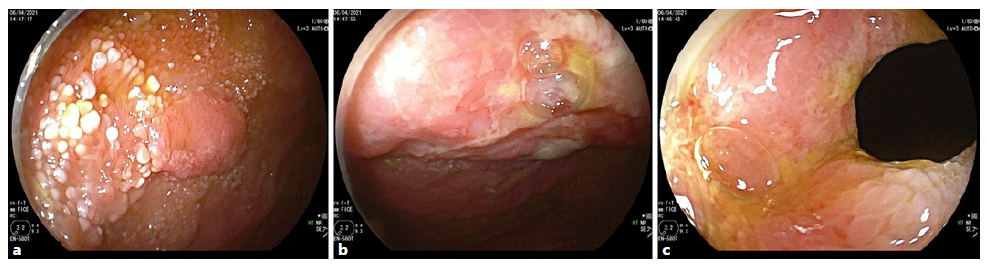
Fig. 2 Double balloon enteroscopy revealed an area of infiltrative appearance at the anastomotic region with features of polypoid component (a), ulceration (b), and stenosis (c) that could not be traversed with the entero-scope.
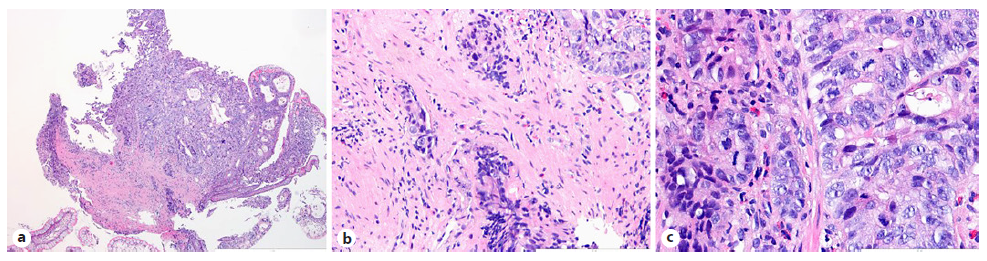
Fig. 3 Histopathological examination of biopsies performed at the region of small bowel ulceration. a Invasive adenocarcinoma with an enteric-type mucosa (HE, ×40). b An area of stromal infiltration by malignant cells is highlighted (HE, ×200). c The neoplasia demonstrates a high mitotic index (HE, ×400).
Discussion
Over the past several decades, it has become increasingly recognized that SBA is a rare but well-known complication of CD. A recent meta-analysis that included 7,344 patients reported that, although the relative risk of SBA in patients with CD was increased 22-fold compared to the general population, the absolute cumulative risk was only 0.23% during a median follow-up of 12.55 years [2]. However, this cumulative risk is directly proportional to the disease duration, and other studies suggest that it increases to approximately 2.2% after 25 years of ileal CD and that SBA accounts for 25% and 45% of the risk of gastrointestinal carcinoma after 10 and 25 years of CD, respectively [6]. Similarly, a prospective observational study demonstrated that the incidence rate of SBA in patients with CD increases from 0.235/1,000 patient-years to 0.464/1,000 patient-years when only patients with >8 years of disease are considered [7].
Risk factors for SBA in patients with CD include ex-tended duration of disease, distal jejunal and ileal location, stenosing or chronic fistulizing behavior, male gender, young age at diagnosis, and the presence of a by-passed small bowel segment [5, 8, 9]. In contrast, a case-control study suggests that small bowel resection and prolonged salicylate use may be protective against development of SBA in patients with CD [10]. Interestingly, the risk of SBA appears to be much higher in patients with isolated ileal involvement than in ileocolonic CD [11]. The risk also appears to be influenced by geographical factors, with a higher relative risk of developing SBA compared to the general population in North America, the United Kingdom, and Scandinavia [11].
Unfortunately, diagnosis of SBA in patients with CD can be quite challenging as clinical symptoms may mimic an acute exacerbation of the disease and imaging findings can be indistinguishable from benign strictures. As a result, most cases are found incidentally after surgical resection for benign indications, and it is diagnosed preop-eratively in only 5% of patients [6]. In a recent retrospective study involving 22 patients with SBA associated with CD, only 2 had a preoperative diagnosis; even for the remaining, where cancer was unsuspected on preoperative assessment, only 25% were diagnosed intraoperatively, whereas 75% were unexpectedly diagnosed postoperatively on final pathology [12].
The most common clinical presentation is with ob-structive symptoms, including nausea, vomiting, and abdominal pain. Less common clinical presentations include hemorrhage, fistula, or perforation [4]. Two important clinical indicators of malignancy include recrudescent symptoms after long periods of relative qui-escence and small bowel obstruction that is refractory to medical therapy [13]. SBA associated with CD usually occurs after a median time of 15 years of CD and is usually diagnosed at a younger age than de novo SBA (median age 47 vs. 68 years, respectively). It is typically found within areas of inflammation of the ileum and jejunum, whereas de novo SBA is distributed all along the small intestine [6].
In general, imaging techniques may miss small lesions and may not be able to differentiate areas of SBA from those of severe CD [4]. Four imaging patterns in CT enterography were distinguished, including small bowel mass, long stenosis with heterogeneous submucosal layer, short and severe stenosis with proximal small bowel dilation or sacculated small bowel loop with irregular and asymmetric circumferential thickening. These findings are nonspecific and may be completely indistinguishable from a benign fibrotic or an acute inflammatory stricture [14]. Magnetic resonance enterography has the advantage of not exposing patients to ionizing radiation and appears to be a useful imaging test for the detection of SBA in patients with CD [15] and a cost-effective approach in patients younger than 50 years old [16].
Nevertheless, cross-sectional imaging does not allow direct visualization or tissue sampling. The small bowel has always been an organ difficult to access by endoscopic procedures. However, in recent years, there has been much development in endoscopic techniques like video capsule endoscopy or balloon/spiral-assisted enterosco-py, which has allowed significant improvement in both the detection and treatment of small bowel lesions [17].
The usefulness of video capsule endoscopy in this setting may be challenged by the stenosing nature of CD (both malignant and nonmalignant strictures) that may result in capsule retention and the inability to obtain tissue samples [18].
Therefore, balloon-assisted enteroscopy appears to be of great value in the evaluation of imaging abnormalities that raise concern for malignancy in small bowel CD. Although balloon-assisted enteroscopy may be limited by invasiveness and incomplete visualization of the small bowel, it presents the advantages of allowing direct visualization and tissue sampling at a low rate of adverse events [19]. In our case, refractory iron deficiency anemia and abnormal imaging findings on CT enterography prompted balloon-assisted enteroscopy, where SBA was discovered. There is another similar previously published case where a 48-year-old man with a 21-year history of CD had SBA diagnosed by PET/CT and double-balloon enteroscopy performed during diagnostic workup for liver metastasis [20], which suggests that more widespread use of balloon-assisted enteroscopy could lead to a more frequent diagnosis of SBA in earlier stages among patients with CD.
There are no formal recommendations on endoscopic screening for SBA in CD patients. In this regard, an exploratory multi-center prospective study involving a cohort of high-risk CD patients defined as long-term small bowel disease without bowel resection was performed and the prevalence of dysplasia and SBA was 4% [21]. Because of its low sensitivity, endoscopic screening cannot be currently recommended. Further studies defining subsets of CD patients at higher risk of SBA that could ben-efit from screening strategies are needed.
Although previous studies suggested that SBA associated with CD was associated with worse survival than de novo SBA [4], this is controversial. A recent retrospective study involving 2,668 patients with SBA did not find significant differences in overall survival between patients with and without CD [22]. These results are supported by another study involving 2,123 patients with SBA, where those associated with CD actually presented at an earlier stage and were more likely to undergo surgery than those with de novo SBA, although no significant differences in overall or cancer-specific survival were found [23]. In contrast, a study that compared SBA associated with celiac disease to SBA associated with CD found a significantly better overall survival in the former group [24]. Prognosis is closely related to disease stage as demonstrated in a retrospective study involving 29 patients with SBA associated with CD, where significant differences in the 2-year survival for node-negative versus node-positive carcinomas (79.3% vs. 49%) and for localized versus metastatic disease (92.3% vs. 33.3%) were reported, as ex-pected [13].
The first-line treatment is wide resection of the small bowel segment harboring the cancer as well as resection of the corresponding mesentery and lymph nodes with right colectomy for lesions of the distal ileum [5]. When surgery is not feasible because of metastatic disease, combination chemotherapy consisting of 5-fluorouracil, leucovorin, and irinotecan with or without gemcitabine may result in prolonged survival, downstaging, and successful secondary complete resection with durable remission [25].
Conclusion
SBA is a rare complication of CD that poses diagnostic challenges. This case demonstrates that clinical presentation may be nonspecific and CT enterography may not be accurate enough to distinguish benign from malignant strictures. Clinicians must, therefore, maintain a high index of suspicion for this complication in patients with long-standing CD with ileal involvement. It is also important to emphasize the role of balloon-assisted enteroscopy, which allowed an early diagnosis. Since early diagnosis has been difficult, a low threshold to perform enteroscopy in high-risk patients, especially those with long-standing ileal CD with refractory or unexplained strictures, may be expected to result in improved diagnostic accuracy, increased detection rates at an earlier stage, and better overall survival.















