Introduction
Inflammatory bowel disease (IBD) encompasses two chronic inflammatory conditions: ulcerative colitis (UC) and Crohn’s disease (CD) [1]. UC is a lifelong disease described by continuous mucosal inflammation involving the rectum and a variable extent of the colon. In CD, the terminal ileum and/or proximal colon are affected in the majority of cases, though the disease may co-exist in other locations [2-4].
Available medical and surgical therapies are more focused in managing the disease symptoms than in a curative result [5, 6]. Although IBD etiology is still clouded, the incidence has been increasing in newly industrialized countries that have become more westernized, with IBD estimated to affect up to 0.8% of the general western population [7-11]. Characterized as a chronic disease with an early onset, low mortality rates and high morbidity, IBD patients require continuous medical assistance, thus creating a considerable healthcare and economic burden [12, 13]. In addition, surgical hospitalizations and the rising utilization of biologic drugs are increasing the cost of treatment for a disease that is continuously increasing its prevalence, exacerbating the burden on the healthcare system. However, while more expensive than conventional therapies, biologic agents are successfully being used to induce remission in patients with moderate and severe forms and unresponsive to conventional therapies, confirming that those are effective alternatives that remarkably improve the overall quality of life [14, 15]. The annual healthcare burden in Europe is estimated to range between 4.5 and 5.6 billion euros, with global disability-adjusted life years (DALYs) of 1,849,068 reported in the Global Burden of Disease study (GBD) [16-18].
The combination of reduced quality of life and a life-long need for medical care places a burden on both patients and caregivers, particularly since onset occurs dur-ing the most economically productive phase of the patient’s life [19-21]. Both UC and CD entail recurring hospitalizations, commuting for appointments and exams, a reduction in work availability and restrictions in time off work. Indirect costs also play a sizable role in the burden of disease [12, 19]. As a result, reports show indirect costs make up almost 50% of the total cost of IBD [17].
To effectively define health policies, improve resource allocation and align patient care health policies for IBD, there is a need to generate data and evidence for this disease. This study aimed to estimate the values associated with the annual health burden and cost of IBD in Portugal.
Materials and Methods
Burden of Disease
The burden of disease was assessed by considering the impact in terms of DALY, a metric adopted by WHO which measures the years of healthy life lost due to disease or premature death. As IBD is a chronic disease, the best suited model to estimate the burden of disease is a prevalence-based model. The most conservative forecast methodology in the paper by Santiago et al. was used to estimate prevalence [22]. DALY is given by, DALY = YLL + YLD, where two time-based indicators are included: years of life lost (YLL), which measures premature mortality due to the disease; and years lost due to disability (YLD), measuring the number of years patients live with disability due to the disease [23-28]. Further details on the methodology used for DALY calculations can be found in the online supplementary Appendix A (for all online suppl. material, see (http://www.karger.com/doi/10.1159/000525206).
Cost of Illness
The cost of IBD was estimated considering the societal perspective to assess the economic impact on the NHS, social security, patients, and caretakers. A prevalence-based model was also used, in which direct costs were estimated for both the NHS and patients in Portugal, alongside indirect costs that could impact other stake-holders. This methodology is commonly used for raising awareness among policy-makers [29]. Both direct healthcare and non-healthcare costs were considered in this analysis. The first entails medical appointments, emergency visits, hospitalizations, surgeries, laboratory tests and exams, drug usage, and administration, and the latter is solely comprised of patient transportation to and from the hospital according to the vehicle used by the patient (see Table 1) [30].
Labor earnings lost due to adverse health disorders are quantified in an indirect cost analysis. Indirect costs are not limited to lost earnings and productivity of patients but also include those of caretakers that assist patients. Costs estimated impact companies where patients are less effective and social security, which assumes the costs of early retirement, sick leave, and work absences by either patients or caretakers.
All costs were calculated separately for UC and CD, except for premature death figures in indirect costs. Further details on the methodology used for cost calculations can be found in the online supplementary Appendix B.
In order to quantify hospital production for IBD patients, the NHS hospital Diagnosis-related group (DRG) database from 2016 was analyzed, since it was the most up-to-date and complete data available, regarding all patients diagnosed with the International Classification of Diseases (ICD-9) codes related to both diseases (555.x for CD and 556.x for UC). All codes used can be found in detail in the online supplementary Appendix C.
Drug costs were analyzed using the consumption of IBD-related molecules in 2019 in both retail and hospital scope. The data sources used were IQVIA’s EHN database (National Hospital Study) for public hospital’s consumption at purchase price and IQVIA’s ICH database (Consumer Health Index) for retail consumption at public selling price. Both databases have national coverage.
A local expert panel, Table 2, composed by 5 Portuguese gastroenterologists’ experts from different hospitals and regions, met for a meeting on December 16, 2019. This panel assisted in filling gaps of information, as described in each section, in order to calculate costs. The panel followed a two-round modified Delphi methodology, where a first round consisted of sending a questionnaire to the experts, followed by a second round of live discussion.
Data collected was not extrapolated since the experts’ view was considered as a representation of the national clinical reality.
To support the cost analysis, an anonymized patient-reported study was conducted online with 370 Portuguese IBD patients. This study was conducted in January 2020 by the Portuguese Association of Inflammatory Bowel Disease (APDI), collecting data from its associates considering a retrospective period ranging between 1 week and 1 year, depending on the type of question. Patients were stratified geographically (NUTS II) and by age. A study error margin of 5% at a 95% confidence level was found [30-36].
Results
Burden of Disease
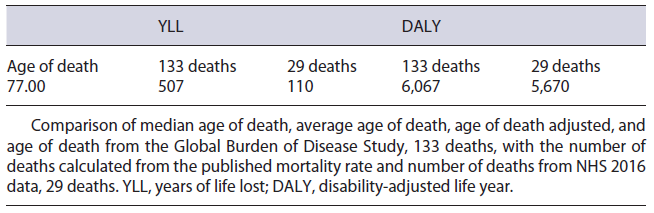
The IBD population in Portugal is estimated to be 24,069 patients. Considering the diseases’ split published, 11,866 of these are estimated to have UC and 12,203 have CD [22, 25, 26]. Considering this prevalence, 6,067 DALYs were estimated to be lost in Portugal due to IBD. This result reflects a total of 507 YLLs and a total of 5,560 YLDs in the Portuguese population. The low value of YLL is a consequence of (1) 133 deaths, calculated using published mortality ratios of 1.19 and 1.38 for UC and CD, respectively [24], and (2) the general population’s average remaining life expectancy of 3.8 years, which was obtained by subtracting the median age of death from NHS 2016 data, 77.0 years, from the average life expectancy in statistical national data of 2018, 80.8 years. On the other hand, the value of YLD was calculated by multiplying the reported prevalence, 232/100,000, by the disability weight stated in the GBD 2017 of 0.231 [18, 22].
For UC, the YLL yielded was 222 as there were 123 deaths in 2016 and the remaining life expectancy was 1.8 years. In turn, for CD, the YLL yielded was 1,117 as the number of deaths was 143 with 7.8 years of remaining life expectancy. On the other hand, the YLD for UC and CD was 2,741 and 2,819, respectively.
Cost of Illness
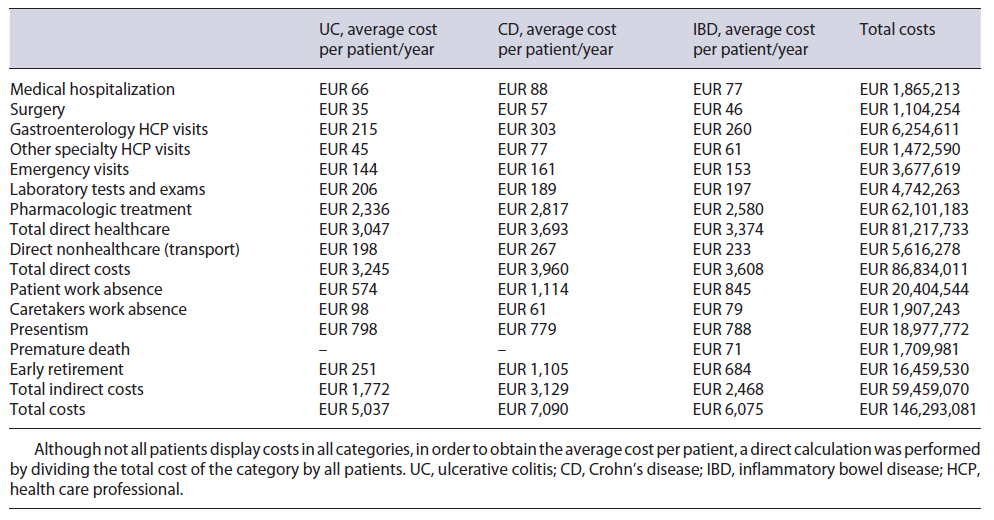
The total annual cost associated with IBD patients in Portugal was estimated to be EUR 146,293, 081, with an average annual cost of EUR 6,075 per patient. This value is divided into EUR 86,834,011 direct costs and EUR 59,459,070 indirect costs. All IBD-related costs are summarized in Table 3, with the respective split between UC and CD. Although not all patients display costs in all categories, in order to obtain the average cost per patient, a direct calculation was performed by dividing the total cost of the category by all patients.
Direct Healthcare Cost
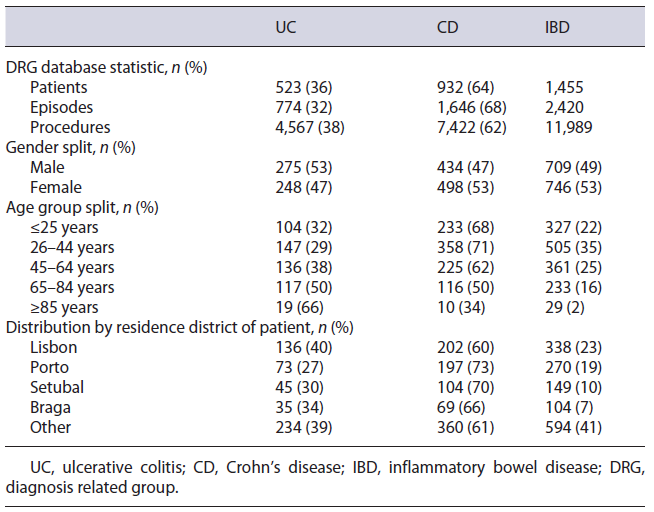
From the NHS 2016 database, 1,455 IBD patients were considered, using the ICD-9 UC and CD specific codes, which accounted for 2,420 episodes and 11,989 procedures. Regarding demography, 82% of patients were under 65 years old, 51% were female, and 42% had their address of residence in either Lisbon or Porto (see Table 4). Medical direct costs resulted in a total of EUR 81,217,733, with an average cost of EUR 3,374 per patient.
Table 4 Key statistics and demographics of the population selected with ICD-9 codes from the National Health Service hospital 2016 database
Medical Hospitalizations
Of the 2,420 episodes registered, 2,148 were recorded as medical episodes. The average cost of a medical episode was EUR 868, meaning a total cost of EUR 1,865,213, with an average of EUR 77.50 per patient.
Surgery
272 episodes out of the 2,420 were registered in our patient data pool as surgical episodes, resulting in an average cost of EUR 4,060 per episode, meaning a total cost of EUR 1,104,254, with an average of EUR 45.90 per patient.
Gastroenterology (HCP) Visits
The 2,420 episodes included were distributed by hospital category, resulting in a price per appointment of EUR 60.20 for UC and EUR 58.10 for CD. With these and the estimate of a total of 94,661 visits (an average of 3.9 per patient - see Table 5), the total cost was calculated to be EUR 6,113,269, with an average of EUR 254 per patient.
Table 5 Number of Health Care Professional visits, emergencies, and exams & laboratory tests per disease and disease stage
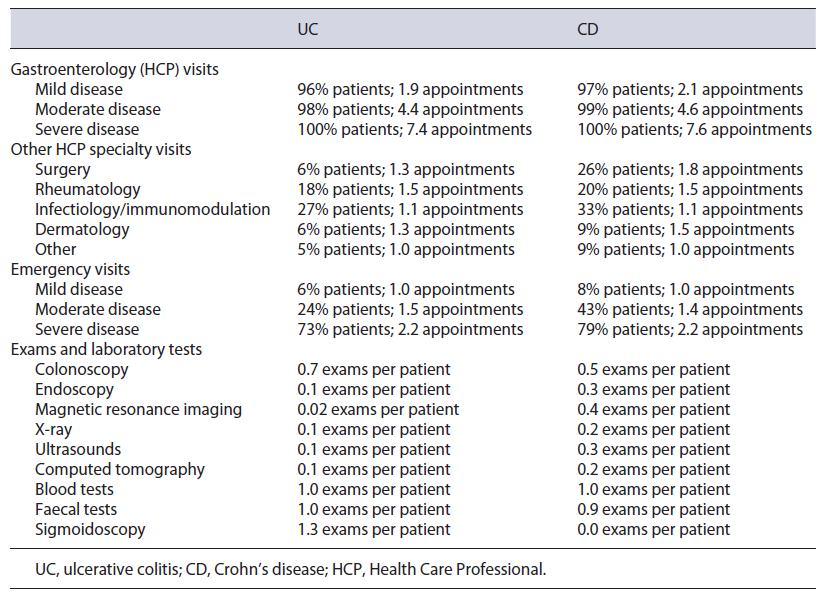
Other HCP Specialty Visits
A total of 25,048 appointments were estimated (an av-erage of 1.0 per patient - see Table 5), and with the same indexed hospital visit price, the total cost was estimated to be EUR 1,613,932, with an average cost of EUR 67.10 per patient.
Emergency Visits
The weighted emergency price was estimated to be EUR 57.70 for UC and EUR 56.60 for CD. While a total of 53,639 emergencies were calculated, 1,198 were already being costed via hospitalization or surgery as this emergency resulted in an inpatient episode, hence only 52,441 emergencies (2.2 emergencies per patient - see Table 5) were costed in this category. Total cost was EUR 3,677,619, with an average cost of EUR 152.80 per patient.
Exams and Laboratory Tests
A total of 99,929 exams were estimated in this category, considering the number of patients and the number of exams per patient (see Table 5). Considering user charge exemptions, a total cost of EUR 4,742,263 was calculated, with an average cost of EUR 197 per patient.
Pharmacologic Treatment
The cost was estimated to be EUR 62,101,183, with EUR 14,995,671 resulting from retail and EUR 46,697,462 from hospital drug consumption. For retail, the split between UC and CD was EUR 11,313,646 and EUR 3,682,025, respectively. This high discrepancy is mainly due to mesalazine (EUR 10,118,105), which is used with considerably greater frequency in UC in comparison with CD. Although the evidence of the effectiveness of mesalazine in CD is very low, the expert panel validated its use in residual cases. In contrast, costs of hospital consumption for UC and CD were EUR 15,295,110 and EUR 31,402,352, respectively. Here, the most decisive parameter is the cost of biologic drugs: 36% of its usage is in UC, while 64% is in CD, shifting the cost weight to CD. Here, certolizumab is referred as being used off-label by the expert panel in a residual number of cases. Immunomodulators administration cost is estimated to be EUR 408,050, with EUR 124,849 for UC and EUR 283,200 for CD. Cost split by molecule in both retail and hospital environment can be found in the online supplementary Appendix D.
Direct Nonhealthcare Cost
It was estimated that 85% of IBD episodes registered, occurred in a hospital within the patient’s district of residence. This resulted in a total transportation cost of EUR 5,616,278, with an average cost of EUR 233.30 per patient.
Patient Out-Of-Pocket Costs
Considering all dimensions where out-of-pocket expenses are present, costs allocated to patients sum up a total of EUR 12,030,257 per year, with an annual average cost of EUR 499 per patient. These costs are included as a subset of direct costs, representing 14% of them.
The patient reported study returned an average of 10% of UC patients and 25% of CD patients that were exempt from medical taxes. As such, the total out-of-pocket cost for HCP visits was determined to be EUR 680,141, with EUR 293,693 for UC and EUR 386,449 for CD. Emergency visits resulted in a slightly higher cost, with a total of EUR 683,595, of which EUR 344,457 were for UC and EUR 339,138 for CD. Exams and laboratory tests out-of-pocket cost amounted to a total of EUR 357,892 for UC and EUR 300,873 for CD.
By considering the reimbursement rates established for each drug, approximately 30% of retail drugs cost was supported by the patients, representing EUR 4,493,975 in co-payments: EUR 3,472,175 for UC and EUR 1,021,800 for CD. Also, according to the patient-reported study, only 3% of emergency visits and HCP visits travels were made by ambulance. Hence, most transportation costs are allocated to patients: EUR 5,513,781 - being EUR 2,336,737 for UC and EUR 3,166,809 for CD.
Indirect Costs
Total costs were estimated at EUR 59,459,070, with an average cost of EUR 2,468 per patient.
Patient Work Absences
A total of 355,539 days were lost due to IBD in 1 year. This number of days lost was estimated by integrating the following four categories:
Inpatient time - a total of 0.7 days per patient were registered in the NHS 2016 database;
Appointment time - considering 0.5 days lost per appointment, a total of 3.6 days per patient were determined to be associated with medical appointments and emergency visits;
Daycare facilities time - following the same assumption of 0.5 days lost per session of day hospital, 0.4 days per patient were calculated;
Sick leave - the patient study recorded 10 days per patient lost yearly as sick leave.
These values computed with the employment rate of IBD patients registered in the patient study, 78.2%, and considering the national daily wage from 2019, correspond to a total cost of EUR 20,404,544, with an average cost of EUR 845.10 per patient. This cost is attributed not only to social security through inpatient time and sick leave but also to either patients or employers since medical appointments and therapy sessions in day care hospital are usually not eligible for sick leave.
Caretaker Work Absences
Patient study responses showed that an average of 34% of patients is accompanied by a caretaker, a cost that is supported by social security, patients’ caregivers, and/or employers. An employment rate of 74% for caretakers and an average of 4.3 days used by the caretaker were also obtained. This yielded a total of 26,055 days, which care-takers lost accompanying patients for IBD-related events, which multiplied by the daily wage resulted in a total cost of EUR 1,907,243, with an average of EUR 79.20 per patient.
Presenteeism
Portuguese IBD patients work an average of 38.3 h per week and the average number of hours lost due to IBD is 2.4 h, thus estimating a 94% efficiency rate when compared to a healthy co-worker. Assuming a total of 220 working days in a year, the patients lost a total of 259,253 days (average of 13.8 days per patient), that amounts to a total cost of EUR 18,977,772, with an average cost of EUR 788.50 per patient. With these costs solely affecting employers, presenteeism is one of the highest indirect costs.
Premature Death
The median age of death for IBD patients reported in the NHS 2016 database was 77.0 years, which differed by 3.8 years from the 80.8 national average life expectancy reported. This number of years lost multiplied by the number of deaths and the average national income per capita resulted in a total cost of EUR 1,709,981, with an average of EUR 71 per patient, a loss supported by social security.
Early Retirement
The number of retirements related to IBD was obtained through the patient study, as well as their average age of retirement - 55 years for UC and 49 years for CD. This data was then compared to the national average age of retirement, which returned a total of 4,881 years lost due to early retirement. Applying the average national income per capita, a total cost of EUR 16,459,530 was estimated, with an average of EUR 683.80 per patient. Like premature death, early retirement costs are supported by social security.
Discussion/Conclusion
This study allowed us to accomplish the proposed main objectives by successfully providing evidence of both the burden and cost of IBD in Portugal, which will allow the filling of an existing information gap at a national level. Considering disease burden, most of the impact of the DALYs lost comes from YLD, which represented 92%, mainly due to the high prevalence of the disease and the early age of onset. On the other hand, the notably small number of deaths and median age of death reported leads to a low YLL, accounting for 8% of total DALYs.
Yet, when comparing the calculated DALYs against the GBD 2017 for Portugal, 4,051, split as 3,029 YLL and 1,022 YLD, there is a significant mismatch not only between final values, but also by split tendency. Being diseases with low reported mortality rates and high overall survival, IBD’s YLD was expected to be significantly higher than YLL. Furthermore, the GBD reported prevalence (6,657) and incidence (4,113) are not in agreement with the values reported in other literature [22, 25, 26]. As such, the DALYs calculated in this report are considered to represent more accurately the current reality in Portugal. Regarding the cost of illness, direct costs of IBD in Portugal represent 59% of the total, while indirect costs, a section usually underrepresented in these kinds of analyses, play a crucial role in IBD.
The major cost driver in direct costs is pharmacological treatment, which accounts for 42% of total costs. This is by far the most relevant category, greatly heightened by the use of biologic drugs. Inevitably, as studies keep providing evidence of the efficacy of biologics with better outcomes, the costs associated with this therapeutic remain significant. Nonetheless, the erosion observed in originators’ prices in the last years, allied to a progressively increasing number of available biosimilars, can mitigate the costs allocated to these therapies in the near future [14, 37]. With the increasing uptake of biosimilar agents at a significantly lower price than the originators, pharmacological costs could be heavily mitigated in the future years [38]. However, with the prediction of a 4-6 fold increase in the prevalence of IBD until 2030 and the major role that biologics play in the treatment of IBD, it is still expected that pharmacological treatment remains the highest cost impact in the next years [22]. Nevertheless, there is clear published evidence that anti-TNF biologics are effective in reducing the odds of hospitalization by nearly 50%, as well as preventing surgery in 33-77% of cases [39]. These outcomes can clearly encourage the use of these drugs, originating savings for the NHS as well as improving patients’ quality of life.
In addition, direct costs calculated in this study are slightly lower than those reported for Portugal in 2004, in which they estimated direct costs per patient to be EUR 3,732 (updated to 2019 prices). Despite being a cohort study with 1,321 patients, the patient pool in Portugal consisted of only 21 patients. Moreover, it did not make any analysis concerning indirect costs, hence the need to evaluate this significant component of the IBD cost [40]. Nonetheless, direct costs are still below what is shown in literature in other countries. In the Netherlands, it is reported that IBD costs EUR 4,866 per patient per year and in the USA, USD 18,637 [41]. Despite these results, data comparison between countries in cost estimation should be interpreted with caution, considering the potential mismatch between methodologies as well as the different cost items considered.
Regarding out-of-pocket costs, the burden for IBD patients is 14% of total direct costs. Even though the Portuguese healthcare system is tendentially charge-free, this share must not be underrated, especially considering that 54% of this value is allocated to user charges and drug co-payments.
Indirect costs account for 41% of IBD’s total cost. Here, the weight is mainly divided between patient work absences, presenteeism, and early retirement. The latter shows that even with a small percentage of early retirements due to IBD-related causes, the impact is still considerable, resulting in a EUR 16,459,530 loss. Interestingly, the costs of patient work absences showed very similar values to those associated with presenteeism. This demonstrates that IBD does not manifest its impact only at isolated timepoints but rather that it is present in the everyday lives of patients, significantly reducing their quality of life. Since there is a significant information gap in the literature regarding indirect costs in IBD, cross-checking these results with other studies is limited.
As both direct and indirect costs play an important role in IBD management, it is important to generate evidence that can support policymakers in allocating resources efficiently. Impact analysis and research must be performed to reduce hospitalization and pharmacological costs, as these results can trigger patient-focused policies and reduce the economic impact of the key cost-drivers.
It is essential to discuss the relationship between the burden of disease and its cost, especially when discussing a clinical condition with an increasing incidence rate in an aging population as it is in Portugal. The cost of a DALY due to IBD is EUR 24,112, which is higher than the EUR 10,999 reported for hemophilia A, the EUR 6,339 reported for atrial fibrillation, or the EUR 15,262 reported for schizophrenia in Portugal [42-44].
This study presents some limitations worth discussing, namely the unavailability of structured data for IBD specifically. The most up-to-date data was used in every possible scenario, requiring an integration of data from different years. Additionally, there are no direct ICD9/10 codes for IBD, hence when using 555.x for CD and 556.x for UC, there is an inherent bias introduced since these may also encompass other conditions. In addition, disease codification errors in the DRG database can occur due to diagnosis misinterpretation of the codifying physicians. Another limitation is that, despite estimating the burden and cost of IBD, we do not account for nonclassified/indeterminate colitis. The NHS hospital DRG database does not include an extensive clinical dataset, which could be used for a more precise clinical characterization of the IBD population and to assess risk groups and additional costs. Also, costs inherent to the use of private hospitals’ admission, surgeries, and drugs were not estimated. In addition to this, in order to better manage the disease, it is common practice among IBD patients to adjust their diet and to seek alternative medicine, which are both not included in the cost analysis.
Regarding the use of prednisolone in retail, there was no way of splitting the consumption allocated to IBD and so a conservative approach was followed, with this molecule not being included in the cost quantification. Regarding IBD mortality, the NHS 2016 data reported 29 deaths. When comparing this value to the published 1.29 mortality rate, a high degree of discrepancy is observed. Taking this into consideration, a two-way sensitivity analysis, summarized in Table 6, was performed to assess the impact of using different variables regarding mortality. Noticeably, despite the parameters chosen, no inversion of tendency is observed, maintaining a YLL value significantly lower than the YLD.
Finally, the IBD pediatric population is not differentiated. All assumptions consider the whole IBD population, characterized by remission, active disease, and disease stages.
In conclusion, as the need to generate further data and evidence regarding IBD arises, this study provides the first comprehensive insight at a national level considering all the dimensions of disease burden. These results will raise social-economic awareness of IBD, allowing for the definition of disease management strategies and support prioritization on resource allocation, especially considering the availability of new treatment approaches. More-over, this study will set the basis for the thorough assessment of the real burden of IBD in the Portuguese healthcare system and society overall.