Introduction
Colorectal cancer (CRC) is one of the most common malignancies in the world, and its incidence and mortality has remained high in the past decades [1], resulting in a pressing problem in today’s society. It has been shown that colonoscopy can be an effective tool to detect and remove the lesions, which exist in the colon and rectum [2]. Additionally, colonoscopy with polypectomy can reduce almost 30% incidence of the overall CRC mortality [3]. Despite all the effort related to screening, it is known that CRC could be diagnosed between the index colonoscopy (in which no cancer was found) and the subsequent colonoscopy [4]. This type of CRC, also called postcolo-noscopy CRC (PCCRC), has been increasing globally, and with population growth, lifestyle change, and diet, PCCRC will be more frequent and common than ever.
According to the World Endoscopy Organization, PC-CRC can be subcategorized into interval cancers and non-interval cancers [4]. Interval cancers are considered when the cancer is identified before the next recommended screening or surveillance examination. On the other hand, non-interval cancers are considered when cancer is identified at (type A) or after (type B) a recommended screening or surveillance interval or when no subsequent screening or surveillance interval for repeat colonoscopy was recommended (type C), up to 10 years after the index colonoscopy [4].
Previous studies have shown that the prevalence of postcolonoscopy CRC - interval subtype (PCCRCi) seems to be less than 10% [5]. Bressler et al. [6] found that the incidence of PCCRCi varies with the location, the incidence being 5.9, 5.5, 2.1, and 2.3% in the right colon, transverse colon, descending and sigmoid colon, and rectum, respectively.
PCCRCi could be correlated with features of colonoscopy (accounting for about 50-75%) and biological features of the lesion (accounting for about 30%) [7]. Factors associated with colonoscopy included: missed lesions that may be the result of inadequate bowel preparation, endoscopist adenoma detection rate, and morphology of the premalignant lesions (flat/sessile adenomas or serrated lesions, mainly when located in the right colon). Besides that, previous studies have shown that about 26% of PCCRCi occur in the same anatomic site where polyp was removed previously [8, 9]. Additionally, Tollivoro et al. [10] have shown that a polyp with more than 10 mm (proximal or distal), adenoma with or without advanced histology, and an incomplete colonoscopy were associated with PCCRCi. Overall, the progression time from adenoma to invasive cancer needs generally more than 36 months [8]. Thereby risk factors for early versus late cancer after a negative colonoscopy (12-36 months vs. >36 months after colonoscopy) included incomplete polyp excision in the colonic segment of the subsequent cancer, failure to examine the segment, and a polyp with 10 mm or more in the segment [10].
With regard to risk factors related to patients, it is known that patients with PCCRCi tend to be older, with a mean age of 74 years [11]. Also, many have family his-tory of CRC [12], history of concomitant disease, history of diverticulosis, and so on.
Although robust and scientifically rigorous epidemiological studies have sifted out clinical and environmental elements linked to PCCRCi, our knowledge of the causes and mechanisms of this group of CRCs is far from complete. In this line of thought, we consider that more attention should be paid to the increasing incidence of PC-CRCi, and more individualized decisions should be made with regard to surveillance range. So, the present study aimed to identify the characteristics and risk factors for the development of PCCRCi, thus being able to contribute to the better knowledge of this group of CRCs, and thus try to reverse the current global trend.
Material and Methods Study
Design and Patients
We performed a retrospective, observational, and single-center study at a Portuguese Reference Center for Colorectal Cancer, in which medical records of individuals who were newly diagnosed with CRC (identified from the national cancer registry) between January 2018 and December 2019 were reviewed. Inclusion criterion was confirmed diagnosis of CRC (based on imaging and/or histological criteria). Patients meeting any of the following criteria were excluded: age <18 years; inflammatory bowel disease; prior diagnosis of CRC; increased familial risk for CRC (two or more first-degree relatives with CRC or at least one first-degree relative with CRC before 50 years); or more than 10 polyps in the index colonoscopy; diagnosis of genetic syndromes that increase the like-lihood of CRC, including Lynch Syndrome and Familial Polyposis. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local Ethics Committee. Informed consent was not requested as this was an observational and retrospective study with no therapeutic inter-vention. The study protocol was authorized by the local ethics committee.
Data Collection
Medical records were reviewed. The following clinical and demographic parameters were considered for analysis: age of diagnosis, gender, residence (rural vs. urban), last colonoscopy and its endoscopic findings, neoplasia characteristics (location, histology, morphology, and staging at the time of diagnosis), family history of CRC, presence of cardiovascular risk factors (CVRFs) (obesity, hypertension, diabetes, dyslipidemia, and tobacco abuse), history of cholecystectomy, the need for surgery, chemotherapy or radiotherapy as treatment options and death directly related to the neoplasia were recorded. All the parameters were analyzed and described for purposes of population’s characterization, not only in the patients with PCCRCi but also among the non-PCCRCi group.
Definitions
Patients with the diagnosis of CRC between two consecutive colonoscopies performed within the appropriated surveillance range were considered to have PCCRCi. The appropriated surveillance range between colonoscopies was defined according to guidelines adopted in the study center during the study period (there were no changes in the guidelines over the time of study period): patients with a normal colonoscopy or with a maximum of two adenomas/serrated adenomas ≤10 mm should repeat colonoscopy within 10 years; the ones with three to four adenomas/serrated adenomas ≤10 mm or with hyperplastic polyp ≥10 mm should repeat colonoscopy within 5 years; patients with advanced lesions (advance adenoma: ≥10 mm or high-grade dysplasia or villous component, including tubule-villous adenomas; advanced serrated adenoma: ≥10 mm; or with any grade of dysplasia); 5 or more adenomas/serrated adenomas or with a traditional serrated adenoma should repeat colonoscopy within 3 years.
PCCRCi is assumed if the diagnosis of CRC has been made within the recommended surveillance period with an extension of up to 6 months beyond the end of surveillance period. All other CRCs were considered non-PCCRCi. The PCCRCi rate was, then, calculated from the ratio between the number of patients with PC-CRCi and the total number of CRC.
The staging of the neoplasia was made in the diagnosis, before neoadjuvant treatment. Location of the neoplasia was divided into two segments: proximal (cecum, ascending, and transverse) and distal (descending, sigmoid, and rectum).
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences® (SPSS), version 24.0. Variables were summa-rized according to their measurement type. Hence, for categorical variables, the authors present frequencies (n) and percentages (%). For continuous variables with symmetric distribution, means and standard deviations were calculated; for continuous variables without symmetric distribution, medians and percentiles P25 and P75 were calculated. The assumption of normality was verified by the Kolmogorov-Smirnov test, through the values of asymmetry and kurtosis, as well as by the analysis of histogram graphs. Chi-square (χ2) tests were used to measure the association between categorical variables, complemented with size effect measures phi (φ) for 2 × 2 tables and Cramer’s v for larger tables.
Multivariate analysis using binary logistic regression was performed to identify predictors of PCCRC. For the purpose of logistic model building, a random sample of the non-PCCRC group, extracted from the data select cases menu in SPSS, was selected in order to accomplish the criterion on 1:3 relation with PCCRC group. Candidate variables for inclusion in a prediction model were any significant (or borderline significant) variables at univariate analysis or variables whose inclusion was supported by the existing literature [13]. Crude odds ratio and adjusted odds ratios (aOR) were determined to measure the effect size of risk factors for developing PCCRC. Bootstrap 1,000 samples were used to model validation and ROC curve to assess the precision of the multivariate risk score. Significance was considered for p < 0.05, except for the purpose of entering predictors in the multivariate model, where p < 0.10 was the threshold.
Results
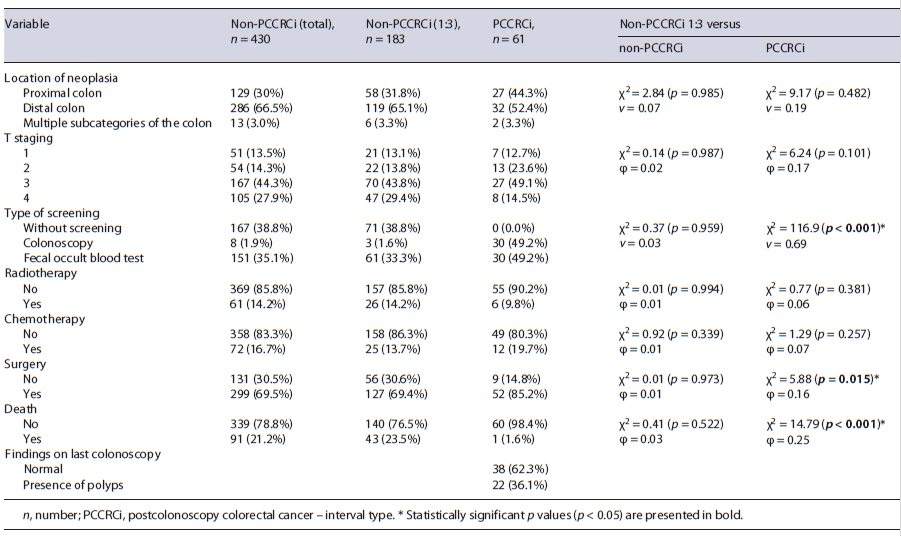
In our study, 491 patients recently diagnosed with CRC were included. Among them, 61 (12.4%) patients had PCCRCi.
For the purpose of logistic model building, a random sample of 183 non-PCCRCi was selected in order to ac-complish the criterion on 1:3 relation. Comparisons were made between the random sample of non-PCCRCi and the original sample of non-PCCRCi and PCCRCi (Table 1). Very similar results were found between the total sample of non-PCCRCi and the random sample, confirming the consistency of the sampling process.
Males were more prevalent for both PCCRCi patients (n = 39, 63.9%) and non-PCCRCi (n = 121, 66.1%), without significant differences between groups (p = 0.756). Mean age was slightly higher in the PCCRCi group (70.13 ± 8.88 years), also without significant differences (p = 0.223). Among patients with PCCRCi, the median time between diagnostic and the index colonoscopy was 5.0 years (P25 = 3.5, P75 = 6.8) with most patients having normal findings in previous colonoscopy (n = 38, 62.3%). Although we did not have access to all quality criteria of the index colonoscopy, all patients had colonoscopies with a bowel preparation allowing complete visualization of the entire colon and rectum.
Surgery was performed on 85.2% of the patients of the PCCRCi group and 69.4% of the non-PCCRCi (χ2 = 5.88 [p = 0.015], φ = 0.16). More deaths occurred in the non-PCCRCi group with 23.5% events, compared to a single event in the PCCRCi group (χ2 = 14.79 [p < 0.001], φ = 0.25), with a mean follow-up period of 2 years.
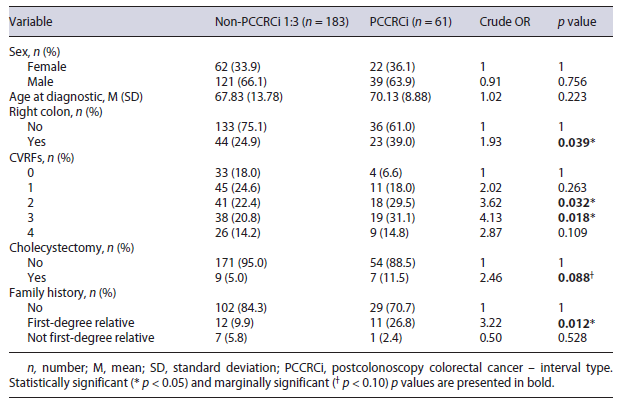
Univariate logistic regressions with crude OR showed an increased chance of PCCRCi for patients with CRC on the proximal colon (OR = 1.93, p = 0.039), two CVRFs (OR = 3.62, p = 0.032), and first degree of family history (OR = 3.22, p = 0.012). For the purpose of entering the multivariate logistic model, we considered marginally significant p values <0.10, thus interpreting as increased chance of PCCRCi for patients with cholecystectomy in the past (OR = 2.46, p = 0.088) as presented in Table 2.
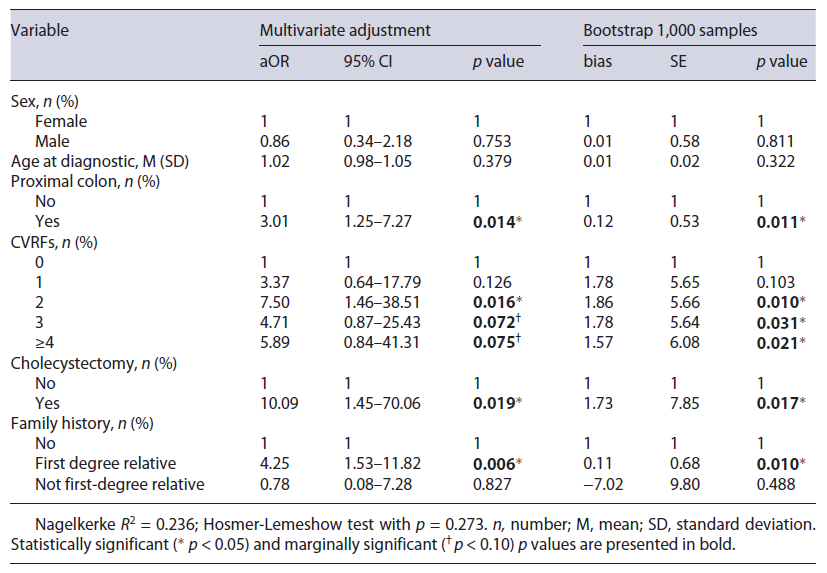
Multivariate adjustment was made, and bootstrap results for all significant and marginally significant previously identified predictors were presented in Table 3. Gender and age were also included as confounders.
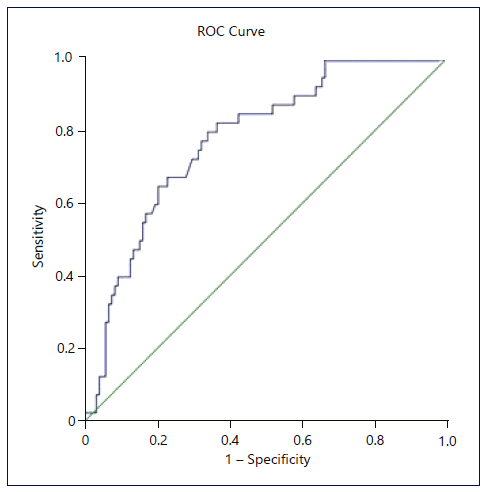
PCCRCi was more frequent in the proximal colon (aOR = 3.01, p = 0.014), and the risk of PCCRCi is associated with the presence of 2 CVRFs (aOR = 7.50, p = 0.016), cholecystectomy in the past (aOR = 10.09, p = 0.019), and family history of CRC in a first-degree relative (aOR = 4.25, p = 0.006). Marginally significant results for the association of PCCRCi with 3 CVRFs (aOR = 4.71, p = 0.072) and 4 CVRFs (aOR = 5.89, p = 0.075). These results suggest that the main threshold for increased chance of PCCRCi is two or more CVRFs. Moreover, isolated CVRF analysis revealed a protective effect for the absence of all CVRFs (aOR = 20; CI 95% 0.05-0.89; p = 0.034). Nagelkerke R2 of 0.236 showed moderate quality. Hosmer-Lemeshow test was nonsignificant (p = 0.273), suggesting a good fit. Bootstrap 1,000 samples were used for model validation confirming significance for the previously identified variables. The ROC curve (Fig. 1) associated with the multivariate model was created, and an area under the curve was 77.8% (p < 0.001) suggesting a moderate precision for the adjusted model.
Discussion
There is no universally accepted definition of PCCRCi, with many studies [14-16] defining durations ranging from less than 1 year to over 10 years after a negative colonoscopy, and are likely to be a heterogeneous group depending on the study definition and population characteristics. This may contribute to the varying prevalence of PCCRCi across studies, ranging from 1.8% to 10% [5, 17]. Our study revealed a higher prevalence of PCCRCi (12.4%), which may support the increasing global trend. Nevertheless, this result should be carefully interpreted considering the single-center, retrospective study design and the longer median time, 5 years, between colonoscopy and PCCRCi diagnosis (most studies calculate PC-CRCi over a period of 0-36 months prior to diagnosis). A more restricted definition could lead to lower PCCRCi rate; however, our intention was to meet current guidelines and therefore bring us closer to real-world perception. A uniform, worldwide definition is essential to minimize the discrepancies among studies. Several studies [6, 11, 12, 18] showed that PCCRCi is more commonly found in female patients and that patients with PCCRCi were older [11, 19]; however, in the present study, neither gender nor age at diagnosis was statistically different in the group of patients with PCCRCi, although the age at diagnosis was slightly higher (70.1 vs. 67.8 years).
In our study, PCCRCi was three times more likely to affect the proximal colon; these results are consistent with a recent meta-analysis carried out by Singh et al. [17], which shows that PCCRCi was 2.4 times more likely to affect the proximal colon than the distal colon. The morphology of the polyps most often detected in the proximal colon (flat adenomas and serrated lesions) may potentially lead to missed lesions, explaining the higher frequency of PCCRCi in the proximal colon [20-22].
Most studies [8, 15, 20, 21] state that PCCRCi mainly develops due to missed lesions, incomplete polypectomy, or eventually new lesions, increasing the risk of CRC in the proximal colon. So, Ferreira et al. [23] emphasize that applying and evaluating colonoscopy quality criteria is essential to assess the effectiveness of screening colonoscopy and should become mandatory in all centers in the near future.
Samadder et al. [12] stated that a greater percentage (57.2%) of patients with PCCRCi presented adenomas at index colonoscopy. Our study showed conflicting results since among patients with PCCRCi, 62.3% had normal findings in previous colonoscopies and only 36.1% presented with polyps/adenomas. This result may be due to colonoscopy related-factors, such as poor-quality indicators that we did not have full access to.
In addition, we found that patients with PCCRCi had a family history of CRC in first-degree relatives, 4.25 times more commonly than the control group, which corroborates the evidence that family history of CRC is more common in PCCRCi and plays an important role in this kind of CRCs as stated in many previous studies [12, 22, 24, 25]. These results further support the idea that some of the PCCRCi may have biological basis (genetic and epigenetic) that further increases the risk of CRC in their relatives, especially those with a family history in a first relative. It also emphasizes the importance of researching the family history of CRC and encouraging adherence to surveillance guidelines in high-risk population.
In other studies, comparing PCCRCi and primary CRC, the presence of multiple comorbidities measured by Charlson Comorbidity Index was associated with PCCRC [11, 17, 19, 22, 26], being in a meta-analysis, the adjusted OR of 2.00 (95% CI 1.77-2.27; I2 = 26%). That said, according to our results, the presence of two or more CVRFs makes the risk of developing PCCRCi 7.5 times more likely. Moreover, though isolated CVRF was not statistically significant for the development of PCCRCi, the absence of all 5 CVRFs seems to be protective. This was the first study to show a direct association between a cut-off of two or more CVRFs and the development of PCCRCi.
Additionally, it is well known that history of cholecystectomy plays an important role in the development of CRC [27-29]. This may be due to the drain of bile acids into the digestive system continuously because of a loss of bile storage and the relaxation of the Oddi sphincter following cholecystectomy and because of the changes in the composition and secretion of bile acids after cholecystectomy [30], thus contributing to the increased risk of developing CCR. Our study demonstrates that the history of cholecystectomy makes PCCRCi 10.09 times more probable, making our study the first to determine it as an independent risk factor for the development of PCCRCi and to introduce it into a predictive model.
Finally, in most other studies [11, 31], staging of PC-CRCi, by the time of diagnosis, was similar or tended to be lower compared with the other types of CRC [12, 17, 19] which is in agreement with our findings. Despite the short follow-up period (2 years), our data reveal a significantly lower mortality rate in the PCCRCi group, which is not consensual in previous studies [11, 17]. However, the earlier stage at diagnosis may correlate with a lower mortality rate in the PCCRCi group given the greater likelihood of curative treatments. More studies are needed to validate the association between earlier stages and lower mortality in this patient group.
Some limitations should be taken into consideration, since this is a retrospective study, in rare situations, some information may have been lost, namely, the presence of CVRFs and the family history of CRC. Also, endoscopic reports were only reviewed from clinical records as most colonoscopies were not performed in the hospital (reports were obtained from the attending physician’s records) and also due to the death of some patients at the time of data collection, which made it difficult to assess some of the quality criteria of colonoscopies (clinical indication for colonoscopy, bowel preparation scale, adenoma detection rate, withdrawal time).
To our knowledge, this is the first study in a western population which found the cut-off of at least two CVRFs and the history of cholecystectomy as risk factors, in a multivariate analysis, for the development of PCCRCi. In conclusion, despite improved techniques for colonoscopy, the incidence of PCCRCi has increased during the past 10 years, showing that there is still much to know about the risk factors and behavior of PCCRCi.
Our data reveal that PCCRCi is more frequent in the proximal colon and identify three independent risk factors for its onset: presence of family history in a first-degree relative, two or more CVRFs, and the history of cholecystectomy. All of them are easily detectable in clinical practice and can be taken into account when defining surveillance intervals. Although the reduction of surveillance intervals, according to the identified risk factors, may lead to a decrease in the rate of PCCRCi, another important problem arises: adverse effects of anesthetic sedation. Therefore, it is essential to combine the patient’s comorbidities, their life expectancy, and the individual need to reduce CRC surveillance intervals. Large prospective, multicenter, and randomized controlled trials are important to certify our findings, validate our prediction model, and attest the efficacy of a more individualized approach.