Dysphagia: Epidemiology, Characterization, and Clinical Relevance
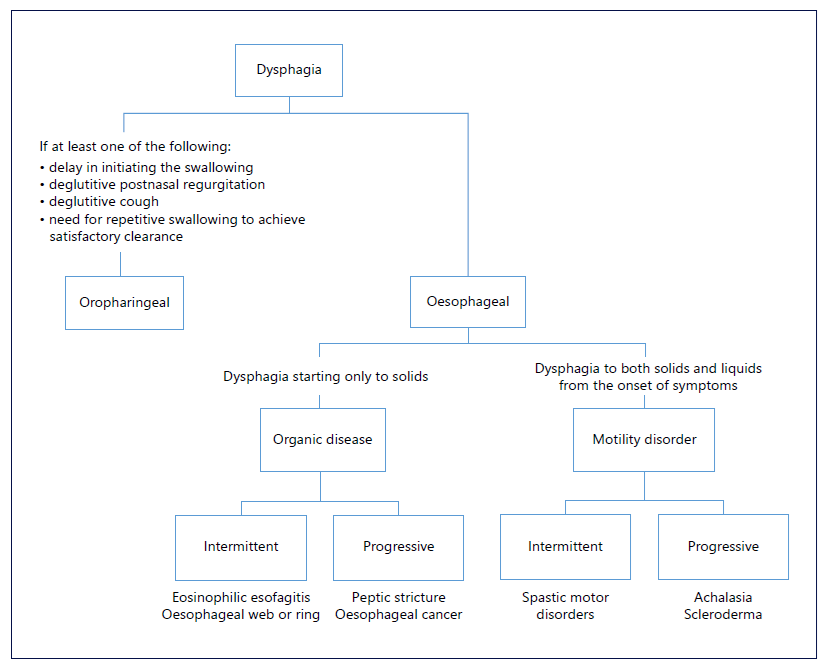
Dysphagia is defined as a subjective sensation of difficulty or abnormality of swallowing [1]. It is a common symptom in the general population, with a prevalence of 20% and affecting up to 50% of people over 60 years [2]. From an anatomical point of view, it can result from oropharyngeal or esophageal etiologies, whereas from a pathophysiological perspective, esophageal dysphagia can be caused by organic diseases (benign or malignant) and functional diseases causing impaired physiology (mainly motility) or perception disorders [3]. A focused anamnesis and knowledge of potential diagnoses will help identify the location and type of dysphagia. To distinguish oropharyngeal from esophageal dysphagia, the site at which the patient experiences the symptom is of limited use since dysphagia felt in the throat can also be referred from the esophagus [2]. There are, however, four aspects that predict oropharyngeal dysphagia with an 80% accuracy: delay in initiating swallowing, deglutitive postnasal regurgitation, deglutitive cough, and the need for repetitive swallowing to achieve satisfactory clearance [2]. Oropharyngeal dysphagia can be associated with neurodegenerative conditions, such as Parkinson’s disease or dementia, as well as with structural causes, namely, Zenker’s diverticulum or osteophytes [3]. Esophageal dysphagia should be characterized according to the types of food that produce symptoms, time course, and associated symptoms. For example, dysphagia to both solids and liquids from the onset of symptoms is probably due to a motility disorder, while dysphagia starting only to solids is usually related to an organic disease leading to a narrowed esophageal lumen. In this last scenario, a progressive evolution of symptoms points out to causes like peptic stricture or esophageal cancer, while an intermittent evolution suggests eosinophilic esophagitis or esophageal ring. On the other hand, patients with motility disorders such as achalasia or distal esophageal spasm (DES) may also exhibit progressive or intermittent dysphagia, respectively (Fig. 1) [2].
Dysphagia is often a life-changing health problem that broadly impacts well being since it can have both physical and psychosocial health consequences. It is associated with many serious complications including malnutrition, dehydration, aspiration pneumonia, and choking, as well as social isolation, depression, and anxiety which also severely reduce quality of life. For patients with dysphagia, mealtimes are often long, exhausting, and difficult and they might feel they cannot eat in the presence of others. This often leads to diminished motivation to eat, sense of social isolation, increased depression, and poverty in overall nutritional intake [4, 5]. Recognition of the clinical relevance and complications of dysphagia is growing among healthcare professionals in several fields. Furthermore, the emergence of new methods to screen and assess swallow function and marked advances in understanding the pathophysiology of these conditions are paving the way for a new era of intensive research and active therapeutic strategies for affected patients [3].
Diagnostic Evaluation of Esophageal Dysphagia
Esophageal dysphagia is considered an alarm symptom, with esophagogastroduodenoscopy (EGD) recommended as the first-line diagnostic test, after a detailed clinical history and physical examination [2, 6]. After excluding structural causes and esophageal mucosal lesions, high-resolution manometry (HRM) is indicated [7]. When both EGD with esophageal biopsies and HRM are normal, a barium esophagogram should be performed to exclude subtle strictures not identified by EGD before considering functional dysphagia [2]. Functional lumen imaging probe (FLIP) has been recently recommended as a complementary tool to HRM in the presence of esophagogastric junction outlet obstruction (EGJOO) or other inconclusive patterns [6, 8]. A cardiac cause should obviously be excluded in a patient presenting with retrosternal chest pain [6]. However, acute dysphagia during a meal, normally associated with retrosternal chest pain, can be due to food impaction, often requiring an urgent EGD [2].
Endoscopy
EGD is mandatory and the first diagnostic tool to be performed in the workup of a patient with esophageal dysphagia, excluding conditions that can lead to secondary esophageal motor dysfunction [6, 9]. Certain findings during endoscopy can also be suggestive of motility disorders, such as increased esophageal diameter and difficulty passing the esophagogastric junction (EGJ) [6]. In the absence of structural lesions, at least six biopsies from the distal and proximal esophagus, in separate containers, should be performed [8, 10].
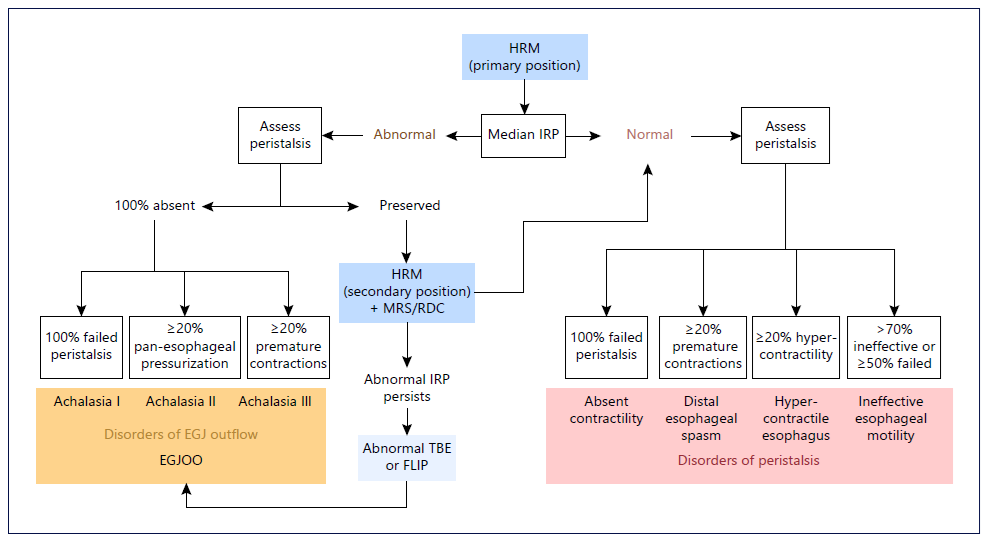
Fig. 2. CC v4.0 for esophageal motility disorders. HRM, high-resolution manometry; IRP, integrated relaxation pressure; MRS, multiple rapid swallow; RDC, rapid drink challenge; EGJ, esophagogastric junction; EGJOO, esophagogastric junction outlet obstruction; TBE, timed barium esophagogram; FLIP, functional lumen imaging probe.
High-Resolution Esophageal Manometry
HRM is currently the gold standard for the evaluation of esophageal motor dysfunction [7]. HRM combined with impedance sensors is recommended to assess intrabolus pressure and bolus transit in relation to manometric pressures [7].
HRM Protocol
The recent Chicago Classification (CC) v4.0 introduced a recommended HRM protocol to standardize the procedure across motility laboratories, in order to optimize generalizability and reliability of HRM interpretation (Fig. 2) [11]. According to the CC v4.0, after HRM catheter placement, the study can begin in either the supine or upright position [8]. A minimum of 60 s of rest after catheter placement allows for an adaptation period, followed by a minimum of three deep inspirations to confirm adequate placement, and subsequently a baseline period of at least 30 s to enable identification of anatomic landmarks including the upper esophageal sphincter, lower esophageal sphincter (LES), respiratory inversion point, basal EGJ pressure, and crural diaphragm [8].
A series of ten 5-mL single wet swallows are then performed with a 30-s interval between swallows [8]. In equivocal cases, the patient is then transitioned from supine to upright or vice versa, again with a 60-s adaptation period, three deep inspirations, and a 30-s baseline period, followed by a series of five 5-mL single wet swallows [8]. Data acquisition in both supine and upright positions has been shown to increase diagnostic yield of esophageal motility disorders [12, 13]. Unless the diagnosis is straightforward, positional changes and provocative maneuvers are recommended, such as multiple rapid swallow (MRS) sequences and a rapid drink challenge (RDC) [6, 7, 11].
HRM Metrics
The key metrics assessed by HRM include evaluation of deglutitive relaxation across the LES (integrated relaxation pressure) and metrics of esophageal body peristalsis based on contraction (distal contractile integral) and latency of deglutitive inhibition (distal latency) [11].
MRS Sequences
MRS is a simple provocative maneuver that consists in the ingestion of five 2-mL swallows in rapid sequence (<10 s) [6]. This test augments central and peripheral deglutitive inhibition, hence suppressing contractions in the esophageal body and inducing relaxation of the LES [6]. The last swallow of the MRS series is followed by a powerful peristaltic sequence in the esophageal body together with a contraction in the LES and reflects the contraction reserve in the esophageal body [6, 7]. An intact response to MRS is defined as the absence of esophageal body contractility with complete deglutitive relaxation of the LES during the repetitive swallows, with an augmented post-MRS contraction [6]. Abnormal results include incomplete inhibition of the EGJ, peristaltic contractility during MRS, or an abnormal contraction after MRS [6, 7]. The failure of post-MRS peristaltic augmentation, as seen in ineffective esophageal motility (IEM), is associated with higher acid exposure time in non-erosive gastroesophageal reflux disease (GERD), late postoperative dysphagia following antireflux surgery (ARS), presence or development of IEM after ARS, and possibly failure of promotility agents [6, 7].
Rapid Drink Challenge
RDC consists in the administration of 200 mL of water and is performed in the upright position and mainly applied for evaluation of EGJ resistance [7]. As with MRS, RDC is not necessary in the majority of cases but is a complementary tool in patients with suspected EGJOO and in achalasia with inconclusive or discordant findings with single wet swallows [7].
Functional Lumen Imaging Probe
FLIP is an assessment tool used for measuring the mechanical properties of the gastrointestinal wall within a specific area, mostly used in the esophagus as a diagnostic device to assess its physiology [6]. FLIP catheter uses high-resolution impedance planimetry during volume controlled distension to measure esophageal cross-sectional area and distensibility [6]. It is nowadays recommended as a complementary tool to HRM in the presence of suspected EGJOO or other inconclusive manometric patterns [6, 8]. The most recent FLIP v2.0 converts the readings to color-coded lumina diameter in real-time plots, enabling evaluation of distensibility index (DI) across the EGJ (measuring the relationship between the cross-sectional area over the distensive pressure to generate luminal distensibility) as well as contractile response to distension in the esophageal body [6, 14]. Studies in healthy volunteers suggest that a normal EGJ distensibility index (EGJ-DI) is >2.8 mm2/mm Hg and normal EGJ diameter is >13 mm [15]. The presence of repetitive antegrade contractions is considered as a normal response to distension [16]. A reduction in EGJ-DI and/or diameter is often seen in patients with EGJOO. EGJ-DI of <2 mm2/mm Hg is considered definitely abnormal, whereas EGJ diameter of <13 mm is likely abnormal and can serve as a supportive measure when EGJDI is indeterminate (2-3 mm2/mm Hg) [15]. FLIP has been demonstrated to predict treatment outcomes and to have a guiding role in therapeutic interventions [17, 18]. Intraoperative use of FLIP in patients undergoing peroral endoscopic myotomy (POEM) resulted in additional real-time myotomy in 65% of cases and improved clinical outcomes [19]. FLIP has also performed superiorly to HRM in the evaluation of bolus emptying [20]. The DI on FLIP has been demonstrated to be a useful measure of EGJ opening in achalasia-treated patients [20]. While the role of FLIP as a first-line tool for evaluation of esophageal motility is still evolving, its role as a supportive test as well as monitoring post-treatment outcomes is increasingly appreciated.
Barium Esophagram
Barium esophagogram consists of the radiographic imaging evaluation of bolus transport through the esophagus into the gastric lumen after ingesting barium contrast, providing information regarding upper esophageal sphincter function, esophageal peristalsis, and bolus clearance through the EGJ [6]. It remains as an important diagnostic test in patients with dysphagia since it can identify structural lesions such as strictures and help identify major esophageal motility disorders [6]. However, the overall sensitivity for the diagnosis of esophageal motility disorders is relatively low (56-69%) [21, 22]. Addition of 13-mm barium tablet to the esophagram protocol along with evaluation of esophageal emptying (timed barium esophagram) at 1, 2, and 5 min can increase sensitivity and also be used to monitor treatment response in disorders of EGJOO [23].
Treatment of Esophageal Motility Disorders
Achalasia and EGJ Outflow Obstruction
Achalasia management is aimed at decreasing the resting pressure of the LES and depends on achalasia type, institutional expertise, patient’s surgical risk, and preferences (Fig. 3). For patients with acceptable surgical risk, pneumatic dilation (PD), POEM, and laparoscopic Heller myotomy (LHM) show similar initial success rates (approximately 90%) and are considered first-line options for type I and II achalasia. In general, type II achalasia responds better to all alternatives [6]. For type III achalasia, POEM is preferred compared to LHM (response rate 93% vs. 71%) as it enables a more precise and longer myotomy, extending above the LES and targeting areas of spasticity, tailored to the findings on HRM [24]. Moreover, POEM is proposed to be advantageous for patients with an anatomically abnormal esophagus (dilated or sigmoid) or refractory to previous conventional treatments [24, 25]. POEM can be performed on both the anterior and posterior sides of the esophagus, with similar efficacy and rate of post-procedural reflux [26, 27]. When compared to POEM, LHM with partial fundoplication comprises higher serious adverse events rate (7 vs. 2%), except for lower reflux esophagitis (44 vs. 29%) [28]. In a recent meta-analysis, including nine randomized controlled trials comparing POEM, LHM, and PD, both POEM and LHM showed a lower rate of treatment failure, followed by LHM in comparison to PD, but neither POEM nor LHM was significantly more effective than the other. There was no significant difference in the rate of adverse events, need for re-intervention or surgery, or GERD. LHM showed the lower rate of GERD and PD the lower rate of erosive esophagitis, with no statistical significance [29]. Another meta-analysis showed that POEM had a significantly better intervention success than LHM but was associated with an increased risk of GERD [30]. To overcome post-POEM GERD, a pilot study including 21 patients was conducted in which an endoscopic fundoplication was added to the standard POEM procedure, demonstrating technical success in all cases without complications [31]. Patients’ comorbidities should be considered when choosing an intervention. Indeed, if a medium to large hiatal hernia is present, LHM with partial fundoplication should be preferred in order to concomitantly correct the hiatus defect [6]. PD presents as the most cost-effective and less invasive procedure, with low rates of perforation (3%) and postprocedural reflux esophagitis (7%) [32-34]. However, its effect weakens over time, with a 90% success rate at 6 months decreasing to 44% at 6 years. For refractory symptoms after initial treatment, if a myotomy (POEM or LHM) was performed, available options include PD or redo myotomy, using either the same or an alternative myotomy technique [35, 36]; if PD was performed, the patient may undergo repeated PD or myotomy. The recently developed EsoFLIP integrates impedance planimetry into a dilator balloon and might prove to be a useful tool in the treatment toolbox of esophageal motility disorders. By providing real-time monitoring of therapeutic dilation as it is being performed, EsoFLIP can possibly enhance performance of dilation by confirming appropriate positioning, but further studies are needed [37]. If the patient is not a surgical candidate, botulinum toxin (BTX) injection should be considered. Injection of BTX into the LES is a simple procedure, inducing immediate symptomatic relief (79%). However, about half of patients need retreatment in less than a year [38]. Moreover, multiple treatment sessions may induce mucosal fibrosis and compromise subsequent interventions [39]. Regarding pharmacological treatment, options include calcium channel blockers (nifedipine) and nitrates (isosorbide dinitrate). However, efficacy is lower and the rate of adverse effects is not negligible, with loss of clinical response over time [6, 40]. Therefore, pharmacotherapy should be used only for patients with achalasia who are not candidates for definitive therapies and have failed BTX injection. EGJOO comprises a heterogeneous group of diseases.
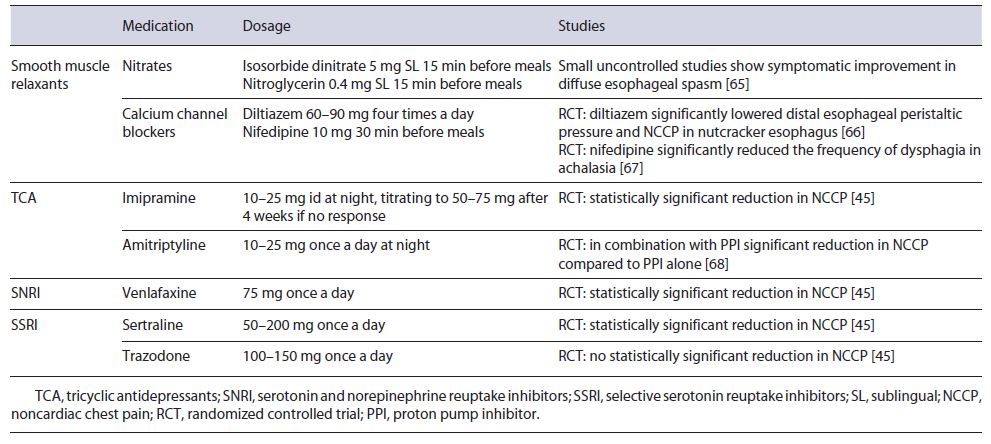
For secondary EGJOO, treatment should target the underlying etiology. Concerning idiopathic EGJOO, it is estimated that most patients with mild symptoms will demonstrate spontaneous resolution [41]. Therefore, treatment is only considered for patients with moderate to severe symptoms and should focus on the dominant symptom. BTX injection is an adequate initial treatment choice. The pooled response rate was 63.6% in six series using the CC v3.0. However, response durability may be limited [42]. Standard endoscopic dilation showed a response rate of 55.6-100% (pooled response rate 69.6%) and PD using a 30-mm balloon or larger showed a pooled response rate of 71.8% [42, 43]. POEM may also have a possible role. In a small retrospective study, POEM was associated with a clinical success rate of 93%, with normalization of integrated relaxation pressure on post-POEM HRM in 71% of the patients with EGJOO [44]. Pharmacological treatments such as smooth muscle relaxants are generally ineffective (pooled response rate 30%) [42]. If noncardiac chest pain is the predominant symptom, tricyclic antidepressants (amitriptyline or imipramine), venlafaxine, and sertraline may be considered [6, 45] (Table 1). Proton pump inhibitors (PPIs) should be used to treat concomitant reflux symptoms.
Spastic Motor Disorders
First-line therapies for spastic disorders (DES and hypercontractile esophagus) include pharmacological treatments such as smooth muscle relaxants and, if noncardiac chest pain is the dominant symptom, neuromodulators may also be effective [6, 45] (Table 1). Furthermore, due to the potential overlap between GERD and spastic disorders, for patients with concomitant reflux symptoms, a trial of PPI is recommended [6, 46] (Fig. 3). For refractory symptoms, empirical PD directed to subtle strictures or luminal remodeling might be an option, with a reported response rate of 70% [47]. BTX injections at the level of the EGJ and at the distal esophagus in patients with spastic disorders have also demonstrated a 1-month response rate of 50% that fell for 30% after 1 year [48]. POEM and surgical myotomy have also been proposed for highly selected patients with spastic disorders with an obstructive physiology. In a meta-analysis, response rates as high as 88 and 72% have been proposed for POEM with extended myotomy in the context of DES and hypercontractile esophagus, respectively, with a low rate of adverse events (14%) [49]. The extended surgical myotomy also demonstrated high clinical efficacy in a subset of 20 patients with DES [50].
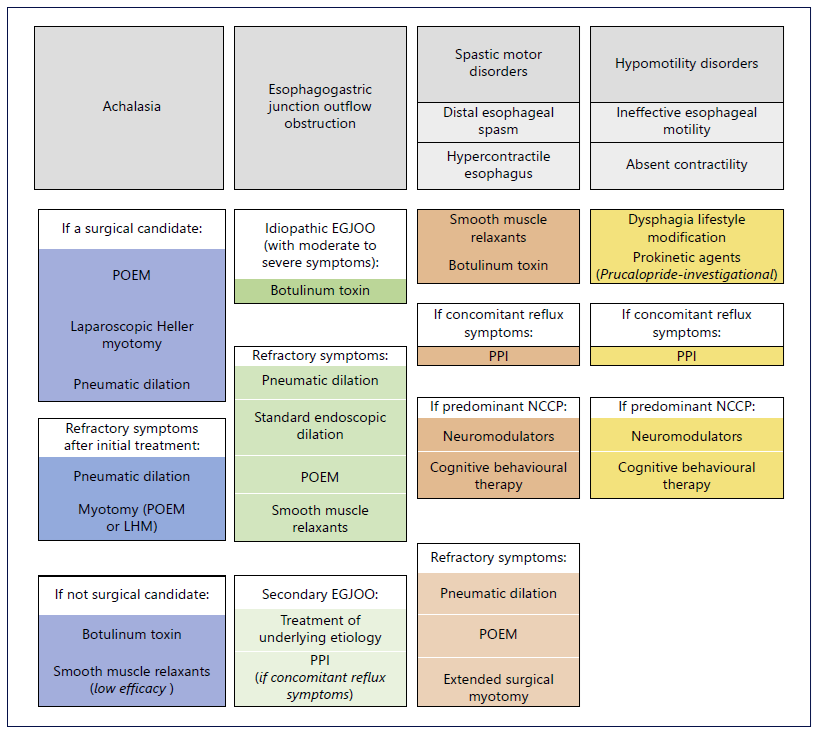
Fig. 3. Treatment options in patients with esophageal motility disorders. CCB, calcium channel blockers; EGJOO, esophagogastric junction outlet obstruction; LHM, laparoscopic Heller myotomy; NCCP, noncardiac chest pain; POEM, peroral endoscopic myotomy; PPI, proton pump inhibitor.
Hypomotility Disorders
There are no drugs capable of restoring esophageal smooth muscle contractility. Moreover, there is no clear indication when IEM needs management since the symptomatic correlation is inconsistent. Diet and lifestyle modification, such as chewing carefully, sitting upright in erect position, chasing solid bolus with liquids, and effective control of GERD, is the mainstay of treatment (Fig. 3). Besides PPI, newer prokinetic agents may prove beneficial. Prucalopride, a selective high-affinity serotonin receptor agonist, approved for chronic idiopathic constipation, increased primary peristaltic wave amplitude in reflux patients [51]. Mosapride, a 5HT-4 agonist, may facilitate secondary peristalsis induced by rapid air distension in patients with IEM, by augmenting sensitivity, but without improvement in primary and secondary esophageal contraction vigor [52]. Buspirone, a mixed dopamine D2 receptor antagonist and partial 5HT-1A agonist, was not more effective than placebo in patients with hypomotility disorders and dysphagia [53]. Metoclopramide and domperidone are not useful [54]. Coping strategies, cognitive and behavioral therapy, and hypnotherapy may be adjunctive therapies [55]. For patients with esophageal hypomotility and GERD symptoms undergoing ARS, except those with absent contractility, complete (Nissen) fundoplication showed similar outcomes compared to partial fundoplication [54].
How to Assess the Severity of Dysphagia (and Select Who to Treat)? The Importance of Patient-Reported Outcomes
When assessing dysphagia, the main goal is to understand the nature of this symptom and its impact on the patient’s daily function [56]. These patients often experience decreased quality of life resulting from impaired social and psychological well-being [57-59]. The idea that patients are able to perceive and report their swallowing difficulty is valuable in the management of dysphagia. Accordingly, a major part of dysphagia assessment relies on subjective measures, collected through the application of validated surveys [56]. Evaluation of dysphagia is challenging. Occasionally, it may cause tremendous distress that patients are not able to effectively describe. On the contrary, they may be oblivious to any swallowing difficulty [56, 57, 60, 61]. Although there are many options available for swallowing assessment, including instrumental and noninstrumental tools, patient-reported outcomes (PROs) have a critical role by providing an accurate patient perception toward dysphagia [58, 60, 62, 63].
PROs are self-assessment tools that capture the patients’ illness experience and help providers better understand symptoms from the patients’ perspective [58, 62, 63]. Regarding dysphagia, PRO can improve clinical outcomes by ascertaining the true individual impact on quality of life. Therefore, these standardized measures are valuable tools for demonstrating treatment effectiveness, directing medical care, and enhancing patient-provider relationship [58, 62, 64]. A systematic review by Patel et al. [62] critically evaluated all dysphagia-related PRO scales for adults. Overall, the dysphagia-related PROs identified demonstrated significant variability in their developmental rigor. There was one general dysphagia PRO measure with exceptional characteristics - PROMIS-GI disrupted swallowing - developed with the goal of evaluating the individual impact of dysphagia independently of etiology or type of dysphagia [62, 64]. Several other high-quality PROs were rigorously developed in specific diseases, namely, FACT-E for esophageal cancer and DSQEoE for eosinophilic esophagitis. One of the most useful applications of PRO is monitoring change in dysphagia over time to compare efficacy of interventions or evaluate the natural history of the condition. However, only a minority of the identified PRO measures demonstrated adequate responsiveness [62]. The relationship between self-perception and objective findings remains to be completely elucidated in dysphagia. PROs complement swallowing assessment, potentially aiding to guide management decisions, also tailored to the underlying etiology [56, 57, 62]. Management of dysphagia is multidisciplinary and involves speech therapists, doctors, nurses, and dietitians. Most importantly, the individual patient should be involved in decision-making throughout assessment and treatment [57, 60, 61]. Naturally, the use of tests cannot replace clinical judgment, which is based on a comprehensive assessment and multidimensional evaluation of dysphagia in a particular individual [57, 63].
Conclusion
Nonobstructive esophageal dysphagia is best characterized by HRM using the hierarchical CC v4.0. Therapeutic approach should be tailored to the underlying condition and considering the impact on patient quality of life. Therefore, PRO may have a critical role by providing an accurate patient perception toward the symptom.