Introduction
Metabolic-associated fatty liver disease (MAFLD) has become one of the most prevalent causes of chronic liver disease worldwide, emerging as the next leading cause of end-stage liver disease, with a global prevalence around 25% [1]. This recent growth in MAFLD prevalence has paralleled the increasing frequency of people with obesity and other metabolic syndrome (MS) components such as arterial hypertension (AH), dyslipidemia, and type 2 diabetes mellitus (T2DM), which is not surprising since these represent the most commonly accepted risk factors for the development of MAFLD [2].
Despite this, there is a heterogeneous pathogenesis in metabolic fatty liver diseases, with inaccuracies in their terminology and definitions precluding clinical trial designs and drug developments. In 2020, a group of experts sought to integrate current understanding of patient heterogeneity captured under the previous acronym nonalcoholic fatty liver disease (NAFLD) and provide suggestions on terminology that more accurately reflects pathogenesis and can help in patient stratification for management [3]. Experts reached consensus that NAFLD does not reflect current knowledge, and “MAFLD” was suggested as a more appropriate overarching term.
The current burden of MAFLD has led to a consequently higher number of referrals to Hepatology Clinic [4]. Although most MAFLD patients do not progress to advanced fibrosis and cirrhosis, there are an increasing number of cases who do develop chronic liver disease and progress to unfavorable outcomes such as hepatocellular carcinoma or liver transplantation [5]. Therefore, a key aspect is to precociously identify patients with a greater risk of clinical progression by worsening liver fibrosis, which might benefit from a closer follow-up and additional treatment with new therapeutic options [6].
A significant turning point in MAFLD is the presence of steatohepatitis (SH), as a result of profound liver cell injury [7]. Liver biopsy remains the gold standard to identify SH, despite being limited by its invasiveness, complications, variability in interpretation, and lack of compliance for seriate monitoring [8]. Noninvasive biomarkers of steatosis and fibrosis such as algorithms, serum biomarkers, and imaging modalities are also widely available but do not measure the degree of inflammatory liver injury [9]. Many different algorithms have been studied in NAFLD; however, only NAFLD fibrosis score and Fib-4 index have been externally validated multiple times with consistent results among different populations and may be used as first-line screening tools to exclude severe fibrosis [10].
In 2020, Newsome et al. [11] proposed to validate a noninvasive score identifying patients simultaneously having SH, elevated NAFLD activity score (NAS ≥4), and advanced liver fibrosis (F ≥ 2). The FibroScan-AST (FAST) score was constructed by combining liver transient elastography (LTE) parameters - both controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) - and aspartate aminotransferase (AST) levels. This score showed good accuracy in reflecting histopathology, providing a novel and efficient way to noninvasively identify patients with MAFLD at risk of clinically relevant SH, with significant inflammatory activity and fibrosis. In this study, our group aimed to identify which metabolic features led to higher values on this newly available score, by applying the FAST score in consecutive MAFLD patients submitted to LTE in our center.
Materials and Methods
Study Design and Data Collection
We conducted a retrospective study including consecutive adult patients with MAFLD scheduled to undergo surveillance LTE for two consecutive years. MAFLD was diagnosed based on the evidence of hepatic steatosis in adult patients (detected either by imaging, blood biomarkers/scores and/or liver biopsy) associated with one of three criteria: overweight or obesity (body mass index (BMI) ≥25 kg/m2 in Caucasian individuals); T2DM; or the presence of at least 2 metabolic risk abnormalities (waist circumference ≥102/88 cm in men and women; blood pressure ≥130/85 mm Hg or specific drug treatment; plasma triglycerides ≥150 mg/dL or specific treatment; plasma high-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/gL in women or specific treatment; prediabetes; homeostasis model assessment insulin resistance score ≥2.5; plas-ma high-sensitivity C-reactive protein >2 mg/L) [12].
Patients were excluded in case of cirrhosis, pregnancy, ascites, liver transplantation, or hepatic surgery. Age, gender, BMI, obesity (BMI ≥30 kg/m2), current smoking habits, T2DM, AH, dyslipidemia data were collected for each patient. Platelet count, AST, ALT, and albumin levels were considered only when blood samples were collected within 6 months of the LTE performance, as validated by Newsome et al. [11].
LTE (FibroScanVR Compact 530®; Echosens, Paris, France) was performed with a minimum fasting of 2 h [13]. LSM and CAP were assessed and expressed in kilopascals (kPas) and decibels per square meter (db/m2), respectively. Measurements were performed by placing the probe covered with ultrasound gel on the right lobe of the liver through 9th to 11th intercostal space on the middle axillary line with the patient lying in dorsal position with the right arm in maximal abduction. An LTE was considered valid if having 10 valid measurements with interquartile range (IQR)/median (M) for LSM below 30% [14]. LTE was initially performed with the M probe in every patient, except those with a skin-capsule distance greater than 25 mm, which was shown to be an independent predictive factor of M probe failure [15]. In those patients with M probe failure, measurements were repeated with the XL probe. Conditions which interfere with LSM measurements reliability, such as extrahepatic cholestasis, aminotransferases ≥5× upper limit of normal, and right heart failure or other causes of liver congestion, were considered to be exclusion criteria.
FAST score was calculated for each patient by inserting LSM, CAP, and AST levels in a formula provided by FibroScan®. FAST score varied on a scale from 0 to 1, with the patients being classified as having low (<0.35), intermediate (0.35-0.67), or high (>0.67) probability of having SH with significant inflammatory activity and fibrosis.
Statistical Analysis
Statistical analysis was performed using SPSS® software, version 23 (IBM, Armonk, NY, USA). Categorical variables are presented as frequencies and percentages and continuous variables as means and standard deviations or median (IQR), when appropri-ate. Reported p values are two tailed, with statistical significance being considered for p value <0.05.
For assessment of each metabolic factor impact on FAST score, univariate analysis was conducted with either student t test/Mann-Whitney test for categorical variables or simple linear regression for continuous variables. Variables with significant (p < 0.05) or nearly significant variables (p < 0.10) were then computed into multivariate analysis by means of a multiple linear regression, in order to identify important contributions in the variability of FAST score values when adjusted for possible confounders. To assess if the above reported variables would predict not only significantly different scores but also being assigned to the high-risk FAST score group, a multivariate analysis was performed by means of a binary logistic regression.
Results
From 128 patients submitted to LTE for MAFLD surveillance, 6 were excluded for not having an AST measurement within 6 months of the procedure and 5 were excluded for not having a valid LTE measurement, with a final sample of 117 individuals. The sample consisted of 62 women (53.0%), with a mean age of 53 ± 12 years. The most commonly found metabolic feature was dyslipidemia (n = 96; 82.1%), followed by obesity (n = 67; 57.3%), AH (n = 55; 47.0%), and T2DM (n = 42; 35.9%). The number of patients simultaneously having these 4 components was 22 (18.8%). Smoking habits were reported in 20 patients (17.1%). As of LTE, median CAP was 303 (IQR 50) dB/m2 and median LSM was 5.5 (IQR 3.1) kPa. Median AST levels were 24 (IQR 17) UI/L (reference levels 15-37). Table 1 summarizes the characteristics of our sample.
Table 1. Patients’ baseline characteristics
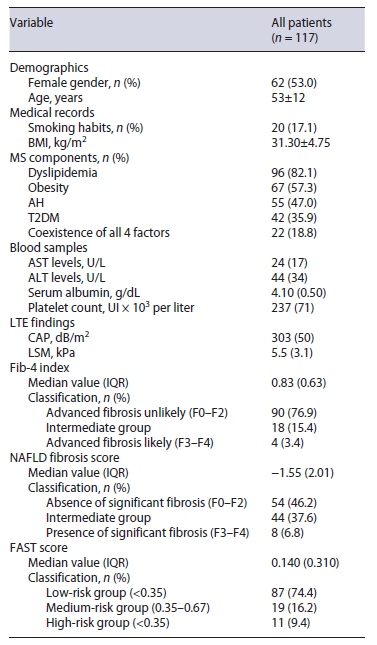
Results are presented in n (%) for categorical variables and mean ± SD/median (IQR) for continuous variables. Dyslipidemia: plasma triglycerides ≥150 mg/dL or specific treatment, plasma high-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/gL in women or specific treatment; obesity: BMI ≥30 kg/m2; arterial hypertension: blood pressure ≥130/85 mm Hg or specific drug treatment; type 2 diabetes mellitus: HbA1c ≥6.5%; fasting plasma glucose levels ≥126 mg/dL, random plasma glucose levels ≥200 mg/dL or specific treatment. AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FAST, FibroScan-AST; IQR, interquartile range; LSM, liver stiffness measurement; LTE, liver transient elastography; SD, standard deviation.
FAST score median value was 0.140 (IQR 0.310). Ac-cording to this score, 87 (74.4%), 19 (16.2%), and 11 (9.4%) patients were assigned to low-, intermediate-, and high-risk groups, respectively.
FAST score had significant moderate correlations to Fib-4 index (r = 0.545; p < 0.01) and NAFLD fibrosis score (r = 0.400; p < 0.01). A total of 8 and 37 patients on the “grey areas” of Fib-4 index and NAFLD fibrosis score would have been reclassified to FAST score high- and low-risk groups, respectively.
Liver biopsy was performed in 23 (19.7%) patients - 4 from the FAST score high-risk group and 19 from the low- or intermediate-risk groups. All of the high-risk patients had confirmed advanced fibrosis and significant SH on the histologic sample, which was significantly different from those in the other groups (100.0% vs. 15.8%; p = 0.004). This represented overall specificity of 100%, sensitivity of 57.1%, positive predictive value of 100%, and negative predictive value of 84.2%.
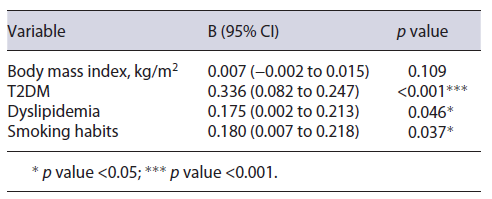
On univariate analysis, the presence of T2DM (0.235 IQR 0.480 vs. 0.100 IQR 0.200; p < 0.001), dyslipidemia (0.165 IQR 0.360 vs. 0.070 IQR 0.120; p = 0.010), and smoking habits (0.305 IQR 0.390 vs. 0.120 IQR 0.280; p = 0.002) resulted in a significantly higher FAST score result. It was additionally shown that patients simultaneously presenting with all four components of the MS presented with significantly higher values when compared to those with 3 or less of the components (0.420 IQR 0.570 vs. 0.120 IQR 0.220; p = 0.001). Male gender (0.170 IQR 0.280 vs. 0.095 IQR 0.300; p = 0.182), AH (0.15 IQR 0.430 vs. 0.125 IQR 0.220; p = 0.512), obesity (0.140 IQR 0.320 vs. 0.140 IQR 0.250; p = 0.851), age in years (β = 0.081; p = 0.386), and body mass index (β = 0.094; p = 0.064) did not show significant associations to FAST score values.
A multiple linear regression concluded that the pres-ence of T2DM (B = 0.165; 95% CI = 0.082-0.247; p < 0.001), dyslipidemia (B = 0.175; 95% CI = 0.002-0.213; p = 0.046), and smoking habits (B = 0.112; 95% CI = 0.007-0.218; p = 0.037) led to significantly higher FAST score values when adjusted for other variables. The model results are described in Table 2.
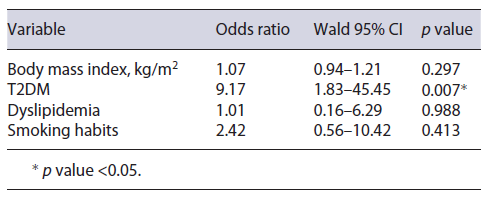
In order to evaluate if these variables would predict not only significantly higher FAST score values but also higher odds of the patient being assigned to the high-risk group, a binary logistic regression was executed with the same predictive variables. After this analysis, only T2DM (OR = 9.2; 95% CI = 1.8-45.5; p = 0.007) was found to be a significant predictive factor of the patient being in the high-risk group. Binary logistic regression results are shown in Table 3.
Discussion/Conclusion
Identification of MAFLD patients with higher risk of progression is of the utmost importance, since a diverse range of therapeutic options, other than lifestyle interventions, is currently under development, particularly for SH [16]. Most MAFLD patients are followed up in primary care centers by general practitioners. Accurate fibrosis assessment in this setting is challenging, since it is limited by performance of liver blood tests, which correlate poorly with fibrosis, and limited access to discriminatory fibrosis tests [17]. Srivastava et al. [18] proposed a primary care referral pathway for patients with MAFLD, where performance of LTE is proposed in cases where Fib-4 index presents with an intermediate result, ultimately concluding which patients benefit the most from specialized hepatology consultation. FAST score gains a crucial role by identifying patients simultaneously having SH with significant inflammatory activity and fibrosis, consequently those who are most likely to benefit from follow-up in specialized centers and to eventually under-go under-development therapies.
FAST score values in our population had significant moderate correlations with indirect markers of fibrosis previously used in NAFLD, namely, Fib-4 index and NAFLD fibrosis score. A correlation was expected, since part of the outcome that the FAST score aims to identify is the presence of significant fibrosis, which is the same predicted outcome in the abovementioned clinical scores. The fact that it was only moderate may be explained as the remaining variability could be attributed to the second outcome in FAST score - the inflammatory activity. By adding this parameter, FAST score could pave its way into clinical practice, as it offers wider information on patients’ disease staging by means of simple and noninvasive diagnostic tools.
Smoking habits represent a classical risk factor for chronic diseases such as cardiovascular diseases, neoplasms, and T2DM [19]. Although far less studied, associations with chronic liver disease have also been reported. Cigarette smoking induces liver disease progression by multiple pathways, the most flagrant one being the induction of hepatic fibrogenesis, to which contributes the systemic inflammation and oxidative stress promoted by heavy smoking [20]. MAFLD is no exception, as was recently shown in a meta-analysis by Akhavan Rezayat et al. [21], where smoking was significantly associated with development of this condition. A key aspect that may help explain this association is the substantial negative impact of smoking in insulin resistance, which is largely accepted to be the main pathophysiologic mechanism in MAFLD development and progression [22]. Moreover, cigarette smoking per se conduces to advanced liver fibrosis independently of T2DM, with nondiabetic patients reporting 10 or more pack-years smoking history having an odds ratio of 2.5 for presence of this adverse outcome [23]. Other than this, recent animal models demonstrated that cigarette exposure, in addition to Western diets, led to significantly higher elevations of biochemical parameters that were accompanied by an increase in hepatic damage shown as more severe fat accumulation, hepatocyte ballooning, and inflammation infiltrates, representing reliable models of MAFLD to SH progression [24]. Our study agreed with previous reports, since patients with cigarette smoking history presented significantly higher FAST scores when adjusted for other variables. In the light of these findings, it is our belief that MAFLD patients should strongly be encouraged to quit smoking, as this represents a modifiable risk factor that can potentially work as a co-factor for progression in addition to other underlying conditions.
Previous reports have already been published mentioning the relationship between dyslipidemia and adverse outcomes in MAFLD, with deranged lipid metabolism being associated with progression to SH [25]. In 2020, a multicentric retrospective cohort of Mexican patients with biopsy-proven SH concluded that high low-density lipoprotein and triglyceride serum levels were the variables with the biggest impact when predicting the presence of advanced liver fibrosis (F4), with an OR of 3.04 and 4.96, respectively [26]. In another cohort study of 260,950 patients, dyslipidemia was one of the significant independent predictive factors of MAFLD/SH progressing to cirrhosis [27]. In our population, similar results were found, as dyslipidemic patients presented with higher FAST score values, reinforcing the urge to implement preventive measures such as nutritional advisory or early statin use in order to achieve control of this component.
The impact on FAST score was rather greater for T2DM, as diabetic patients were also more frequently classified as being in the high-risk group (FAST score over 0.67; OR = 8.26; p = 0.012). A bidirectional associa-tion between MAFLD and T2DM has already been consistently described in literature [28]. First, MAFLD patients have higher insulin resistance rates than those without MAFLD, regardless of body mass index and whether already having T2DM or not [29]. For that reason, MAFLD patients present a 2-fold increased risk of developing T2DM [30]. On the other hand, patients diagnosed with T2DM present with 80% more liver fat than age-, weight-, and sex-matched nondiabetic patients [31]. This difference remains significant for any given body mass index or waist circumference, according to the authors’ findings. Our results were in line with previous reports, as patients with TD2M presented with significantly higher FAST score values and therefore simultaneously higher inflammatory activity and significant fibrosis. The key aspect for T2DM is that in addition to having higher scores, these patients had a significant 8-fold increased risk of being assigned to the high-risk group, differently from the other mentioned conditions. These findings strengthen the important role of T2DM in MAFLD, with this causality already acknowledged in the European Association for the Study of the Liver guidelines, by recommendation of MAFLD screening in all T2DM patients regardless of transaminases levels, as these patients are expected to be at n higher risk of disease progression [32]. Our group believes that TE, with further application of the FAST score, may play a crucial role in this setting, by precociously identifying diabetic patients who are more likely to benefit from biopsy for trial enrollment or subsequent treatment. An investigation by Ciardullo et al. [33] has already shown that, in hypertense patients, T2DM was a factor that significantly increased referral for specialized hepatology consultation due to MAFLD . Therefore, these patients should be promptly referred to specialized hepatology consultation, so a rigorous follow-up program can be achieved. Nonetheless, nondiabetic patients with MAFLD must also be encouraged to maintain healthy lifestyles and advised on dietetic measures in order to evade T2DM development.
Classically, obesity has been accepted as the main risk factor for MAFLD development [32]. This association is explained not only by higher amounts of visceral fat, and therefore liver fat, in overweight and obese people, but also because these patients are more likely to have other MS compounds such as AH, dyslipidemia, and T2DM [34]. Additionally, obesity also increases the risk of having a more histologically severe disease, with the prevalence of SH rising from 2.7% in lean individuals to around 27% in morbidly obese patients undergoing bariatric surgery [35]. So, it may seem surprising that, in our sample, neither obesity nor body mass index values resulted in significant differences on FAST score values. However, this can be explained as most of the reported investigations did not adjust obesity impact for its comorbidities, which can result in bias since, as stated before, those patients more frequently have other risk factors such as dyslipidemia and T2DM. Supporting our findings is an investigation published in 2020 by Lum et al. [36]. From a population of 263 adults with biopsy-proven MAFLD, the development of SH and the presence of significant fibrosis was not significantly different between obese and nonobese patients. Knowing this, every clinician must be aware that lean MAFLD patients are as susceptible as the obese ones to present with important liver disease. Thus, comparable or even tighter caution must be taken when managing this subset of individuals.
A note must be made on the fact that a synergism effect was seen in our population, as patients with combined T2DM, obesity, AH, and dyslipidemia presented with significantly higher FAST scores when compared to those with 3 components or less. Caution must be taken when managing this set of patients, and therefore our group suggests their follow-up to be ideally kept at specialized consultation, as these patients are expected to benefit the most from additional treatments.
In conclusion, our study represents a groundbreaking evaluation of MAFLD in a Portuguese population. In the last years, few studies have addressed MAFLD in Portuguese patients. In 2020, Leitão et al. [37] have analyzed the prevalence and risk factors of fatty liver in a random sample of Portuguese adults, having found an overall prevalence of 17.0%, with MAFLD individuals being more frequently older and with increased probability of having obesity or diabetes. Nevertheless, fibrosis assessment and risk factors for significant fibrosis were not measured. More recently, in 2022, Rigor and associates have validated different noninvasive fibrosis tools in a Portuguese MAFLD sample, which presented an overall advanced fibrosis incidence of 21.5% [38]. However, the newly available FAST score was not yet applied in this population. Therefore, we believe our investigation represents a breakthrough evaluation, by not only being the first to apply the FAST score on a Portuguese population but also by evaluating the weighted influenced of each MS component on the assessed outcomes.
Our investigation has few limitations, namely, its retrospective design and the unavailability of gold standard comparison with liver biopsy in every patient. Nevertheless, our conclusions pave the way for further validation with prospective multicenter studies with larger samples, which will allow a better comprehension of this newly reclassified definition of MAFLD.