Introduction
The introduction and widespread implementation of endoscopic ultrasound (EUS) as a minimally invasive therapeutic modality has garnered significant attention in recent years. EUS has significantly revolutionized the field of interventional gastroenterology. The European Society of Gastrointestinal Endoscopy (ESGE) advises that these therapeutic EUS procedures be performed by experienced endoscopists at centers with adequate multidisciplinary support [1, 2]. In this state-of-the-art review, we will highlight the current indications for therapeutic EUS, including drainage of hepatobiliary and pancreatic obstruction, ablation of pancreatic cysts, management of gastric varices (GVs), and gallbladder drainage.
Methods
We conducted a literature search across three databases (PubMed, Embase, and the Cochrane Library) up to November 2022. The research topics were prepared by the senior author (T.H.B.) and the literature search was performed by the first author (A.C.). Topics included drainage of hepatobiliary/pancreatic obstruction, pancreatic cyst ablation, GVs management, and gall-bladder drainage. All study types were included (randomized controlled trials, retrospective, prospective, meta-analyses, case series, and case reports).
EUS Therapy for Biliary Obstruction
In instances of benign and malignant biliary obstruction, endoscopic retrograde cholangiopancreatography (ERCP) with transpapillary stenting remains the first-line management option [3-6]. In expert hands, ERCP has a success rate of up to 95% with an adverse event (AE) rate <10% [7, 8]. Difficult, failed, or impractical cannulation can be attributed to surgically altered anatomy (SAA), prior duodenal stenting, tumor obstruction, or periampullary diverticulum/tumor [3, 6]. Furthermore, difficult cannulations are also associated with higher rates of AEs, especially post-ERCP pancreatitis (approximately 5.3-6.6% of all cases) [9, 10]. In the setting of unsuccessful ERCP, current guidelines recommend reattempting the procedure at least two to 4 days later in order to optimize success by improving biliary visualization (decreased edema), patient sedation, and availability of specialized guidewire equipment [3].
Historically, percutaneous transhepatic biliary drainage (PTBD) has been utilized as salvage therapy in the event of ERCP failure. PTBD is associated with a high success rate (95%); however, AEs are not uncommon (up to 30%), and the presence of an external catheter has also been associated with a reduced quality of life [6, 11]. It is in this setting where EUS-guided biliary drainage (EUS-BD) evolved as an alternative minimally invasive approach. The first reported case of EUS-guided bilioduodenal anastomosis was performed in 2001 [12]; since then, there have been a multitude of studies describing various techniques to accomplish EUS-BD. Compared to PTBD, EUS-BD is associated with fewer AEs and unscheduled reintervention rates with similar rates of success [13-15].
As such, EUS-BD has emerged as a reliable alternative when ERCP is not feasible [5]. Currently, performance of EUS-BD is limited to high volume centers driven by local expertise. The endoscopic learning curve is linked to procedural volume whereby technical success, procedure time and decreased AEs dramatically improve with operator experience [16-19]. It has been suggested that 33 and 100 cases are needed to achieve technical proficiency and mastery, respectively [18, 19]. The authors of this review article recently published a large single-center study (all procedures conducted by Dr. Baron) of EUS-guided transhepatic biliary drainage where total AEs (18.6%) significantly decreased over the 7 year time period in a cohort of over 200 patients [20]. That being said, EUS-BD is still a challenging procedure, largely limited to tertiary centers where there are rare instances of ERCP failure. One study found that ERCP failure in native papilla occurred in 0.6% (3/524 cases), in which all 3 patients were successfully managed by EUS-BD [21]. Yet there is growing evidence that EUS-BD can be considered as a first-line approach.
EUS-BD Techniques
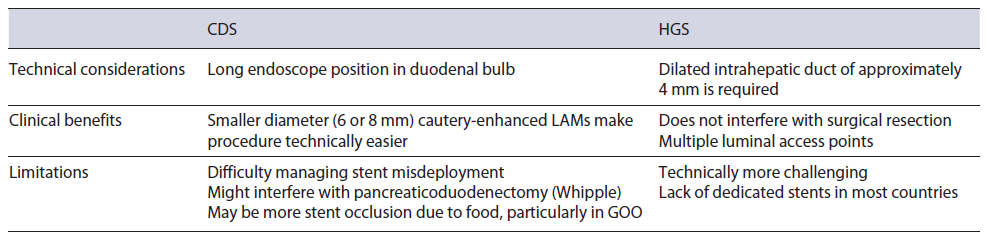
Before delving into comparative studies, it is first important to describe the methods of biliary decompression, which can be achieved through rendezvous (RV), antegrade or transluminal approaches [22]. EUS-RV is limited to cases where the papilla can be reached and used as salvage therapy when conventional ERCP fails, whereby guidewire is accessed through the papilla in an antegrade fashion [22]. This approach is associated with a success and a major AE rate of 80% and 11%, respectively [22]. In RV, the puncture site (via transgastric into left intrahepatic duct or transduodenal into the extrahepatic duct) enables guidewire placement across the stricture/papilla without fistula tract formation [2]. Antegrade stent placement involves transhepatic puncture, passage of a guidewire across the obstruction, and passage of a stent antegrade across the obstruction such that the entire stent is within the biliary tree. If technical failure occurs, antegrade stenting can be converted to transmural or PTBD [2]. In general, direct transmural drainage is preferred using a hepaticogastrostomy (HGS) or choledochoduodenostomy (CDS) approach. Antegrade stenting, performed by placing an internal stent transhepatically, has fallen out of favor as it can be cumbersome with only a 77% technical success rate [22].
HGS typically involves creating an anastomosis between the lesser curvature of the stomach and a dilated left intrahepatic duct using a partially or fully covered self-expanding metal stent (SEMS) (Fig. 1) [23]. Meanwhile, CDS involves tract formation between the duodenal bulb and common bile duct with placement of a SEMS or lumen apposing metal stent (LAMS) (Fig. 2) [24]. Of note, luminal access points for transhepatic biliary drainage can also include the esophagus and jejunum [20].
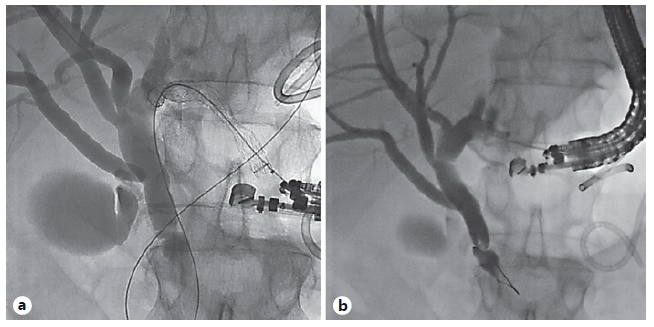
Fig. 1 EUS-guided hepaticogastrostomy in a patient with necrotizing pancreatitis and biliary obstruction due to extrinsic compression, failed ERCP due to duodenal obstruction. a Initial puncture through gastric wall and cholangiogram showing distal bile duct obstruction. b After placement of transgastric fully covered self-expandable metal biliary stent into left hepatic duct.
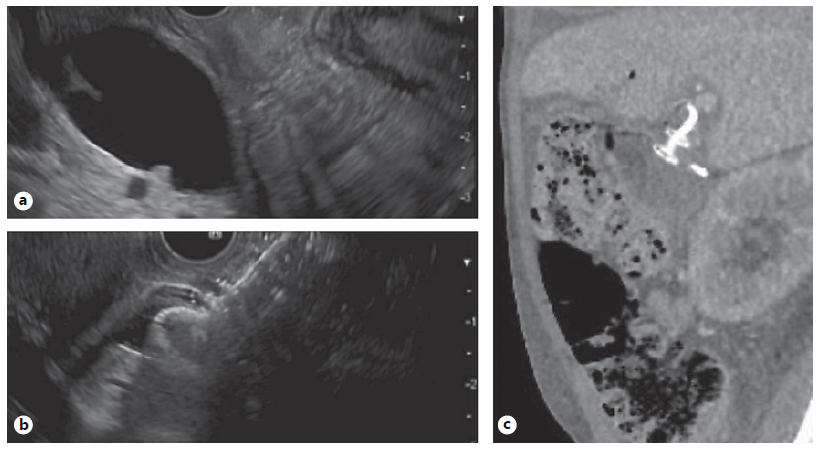
Fig. 2 EUS-guided choledochoduodenostomy in a patient with malignant biliary and duodenal obstruction. a Echoimage of markedly dilated CBD prior to placement of luminal apposing metal stent. b Echoimage immediately after deployment of 8 mm diameter luminal apposing metal stent into distal CBD. c Follow-up CT for continued care. Sagittal image shows luminal apposing stent with plastic stent within at site of choledochoduodenostomy.
Transhepatic and transduodenal drainage methods have been extensively compared [25-36] with similar rates of technical and clinical successes based on three recent metaanalysis [37-39]. A recent international multicenter study of 182 patients (HGS 95 vs. CDS 87) found that technical success was 92% in both groups, while clinical success was slightly higher in the CDS cohort (100 vs. 86%) [36]. The authors found that CDS was associated longer term stent patency at the expense of slightly higher AEs [36]. Another multicenter randomized trial comparing HGS (n = 24) to CDS (n = 23) reported a technical success rate of 100% and 95.7%, respectively [35]. They found no differences in stent patency or AEs. Based on their results, the authors felt that switching between either procedure can be considered when technical challenges arise.
At the moment, there is no standardized algorithm between the two techniques. EUS-guided transhepatic drainage is the first-line method for patients with surgically altered anatomy or hilar obstruction (Table 1). Our recent single-center retrospective study of 215 patients primarily utilized a transgastric approach in 188 cases, where we reported a technical and clinical success rate of 85.3% and 87.25, respectively [20]. Advantages to transhepatic drainage are that in the event of complete stent misdeployment, the peritoneal space involving HGS may be easier to manage in the event of emergency surgery [20]. Additionally, the HGS location may allow for easier surgical resection of the duodenum (during Whipple operation for pancreas head cancer) in operative candidates. We do recognize that this is a technically difficult procedure and use in the community is likely impracticable.
There is evidence supporting the use of EUS-BD as the primary method for biliary decompression in instances of malignant biliary obstruction [22]. Three randomized controlled trials comparing EUS-BD to ERCP found no major differences in technical or clinical success rates [40-42]. That being said, a meta-analysis of these studies found that EUS-BD was linked to a lower rate of stent dysfunction, which leads to less interruption in oncological treatment plans [43]. Similarly, a recent meta-analysis of 5 studies involving 361 patients, found that ERCP was associated with higher rates of reintervention (22.6 vs. 15.2%) and tumor overgrowth (odds ratio 5.3) [44]. While there was no difference in overall survival, one study showed that quality of life was higher in the EUS group [41]. At the moment, larger comparative studies are needed to determine if avoiding transpapillary stenting can influence oncologic treatment outcomes.
EUS Drainage of Pancreatic Ductal Obstruction EUS-guided pancreatic duct drainage (EUS-PDD) is a technically complex procedure associated with a high rate of AEs [2]. It is indicated when ERCP fails (3-10% of cases) or is not possible in cases of SAA [1]. While surgery is superior for long-term symptomatic relief of chronic pancreatitis [45], not all patients are ideal operative candidates and some may prefer a minimally invasive alternative. Main pancreatic duct (MPD) obstruction can result from chronic pancreatitis, pancreatojejunostomy anastomotic strictures, congenital anomalies, or disconnected pancreatic duct syndrome; these etiologies can result in significant patient discomfort and/or bouts of acute recurrent pancreatitis due to underlying ductal and interstitial hypertension [46]. At this juncture, EUS-PDD can be utilized to provide decompressive therapy.
The two approaches include RV-assisted endoscopic retrograde pancreatography (RV-ERP) or EUS-antegrade. In terms of safety and efficacy, RV-ERP is favored, while an antegrade approach is typically employed when RV-ERP is technically unsuccessful or not possible [1, 2, 47]. A 19-gauge needle is preferred to create a transgastric to MPD access point - the MPD diameter should be ≥4 mm [2]. In cases of SAA, a transenteric route can also be used [46]. Assuming there is an endoscopically accessible native papilla (or anastomosis), a guidewire can be passed in an anterograde fashion in order to perform a ERP. Compared to a transmural approach, the RV-ERP method preserves anatomy and may provide better physiological drainage of the ductal stricture [1, 48]. Furthermore, avoiding the need for thermal energy or tract dilation to create a fistula may reduce the risk of bleeding, pancreatic leakage, and gastric leakage into the retroperitoneal space [1]. Yet, when direct drainage is required, cautery and non-cautery transmural stent placement can be accomplished via an EUS-antegrade fashion.
While there are limited head-to-head studies, RV-ERP is universally considered the first method used followed by EUS-antegrade. A large retrospective study demonstrated improved technical success with ERP-RV (95.6%) versus transgastric pancreaticogastrostomy (77.8%) with an added benefit of a lower rate of AEs [49]. As salvage therapy, antegrade drainage has demonstrated pooled technical and clinical success rates of 89% and 87%, respectively [46]. The critical step of creating a pancreatico-gastrostomy is dependent upon successful stent placement through the tract. There are no standardized techniques, though we prefer to use a 19G needle, a 0.025’’ diameter × 450 cm long biliary guidewire, avoidance of cautery whenever possible, and least possible dilation of the tract to achieve the desired stent placement. In terms of stent placement, plastic stents are preferential due to ease of placement, which may reduce AEs in the event of stent dislodgement [50]. In one study fully covered SEMS were placed in 25 technically successful cases; no stent migration occurred and mean stent patency was 127 days [51]. There has been a case report using LAMS and a double-pigtail plastic stent [52], though more data are needed to determine patient selection. A recent study utilized a technique to reduce the risk of leakage and stent migration by dilating the pancreaticogastrostomy tract to 4 Fr using an angioplasty balloon and placing a 3-Fr stent with the pigtail in the pancreatic duct and the straight end extending at least 3 cm into the gastric lumen [53]. The authors reported an 88% technical and 62.5% clinical success rate with no instances of stent-related AEs [53].
While the optimal stent type is being investigated, the need for antegrade stent exchange following pancreaticogastrostomy is still debated among centers. Since drainage is occurring through the gastric wall without an intervening stricture, the need for repeat stent exchange of the MPD can be determined based on clinical/radiographic features or as a standard caliber upsizing procedure after the index endoscopy. An early retrospective study of 36 patients undergoing EUS-guided pancreatogastrostomy and pancreatobulbostomy found that 55% of patients experienced stent obstruction or migration over a median 14.5-month follow-up period requiring 29 repeat endoscopies [54]. Based on their findings, the authors recommended a proactive stance for stent exchange/upsizing using the existing transmural fistula. However, another group found that only 15% (4/26) of their patients experienced stent dislocation over a median follow-up of 9.5 months [55]. The authors supported watchful waiting in the absence of symptoms and radiologic confirmation of stent placement. A recent technical review recommended an elective stent exchange in order to widen the fistula as means to facilitate additional endoscopic therapy through the tract [46]. In light of these different approaches, we believe that the existing tract can be used ≥4 weeks after the initial procedure when the tract has matured [56].
The technical difficulties related to antegrade approach limit its use to expert centers. One study suggested that the learning curve for efficiency (i.e., reduction in procedure time), and proficiency were seen following the 27th and 40th cases when performed by a single operator [57]. However, these results are not generalizable given the expertise of that highly experienced endoscopist. With the current available studies, the AE rates of EUS-PDD range from 12 to 15% [46, 58]. The majority of these consist of abdominal pain, bleeding, infection, pancreatitis, and perforation and are recognized immediately or early post-procedurally [2]. While some studies have reported higher AEs than cited above, the heterogeneity of patients, and use of varying equipment and techniques make it difficult to compare and analyze these findings [46]. Moving forward, we believe that pancreatic ductal drainage may evolve to the use of small-diameter CMSEMS (6 mm) that will reduce the risk of leakage and bleeding.
EUS-Guided Gastroenterostomy for Gastric Outlet Obstruction
Malignant gastric outlet obstruction (GOO) is a mechanical obstruction that can extend from the pylorus or proximal duodenum to the third duodenum. Symptoms range from early satiety to intractable nausea, vomiting, and abdominal pain which result in nutritional deficiencies and poor quality of life [59]. As a result, these patients may also experience significant delays in administration of chemotherapy. Traditionally, bypassing this obstruction was achieved with a surgical gastrojejunostomy (S-GJ) or placement of an enteral self-expandable metal stent (SEMS). However, both methods are somewhat limiting. While a S-GJ can provide longer palliation than SEMS, its use is offset by the high morbidity and mortality associated with surgery in already frail patients with a poor performance status [60]. Meanwhile, enteral stenting (with SEMS) can produce comparable clinical results, yet these benefits are short-lived due to due to recurrent obstruction that occurs in 50% of patients within 6 months [59, 61]. The goal for managing GOO is to relieve the obstruction and allow patients to resume peroral intake.
In this context, the application of EUS-guided gastroenterostomy (GE) is a safe and effective, minimally invasive alternative with comparable outcomes and fewer reinterventions compared to enteral stenting [62] and S-GJ [63], when performed by expert endoscopists. This is especially beneficial for patients with end-stage malignancy who are not surgical candidates. A handful of studies have explored outcomes in both benign and malignant GOO with technical and clinical success rates ranging from 87 to 100% and 84 to 92%, respectively [30, 63-66]. The ability to provide durable symptomatic relief may also be en-hanced by the use of larger LAMS (20 mm) which may decrease the risk of reobstruction and allow patients to tolerate a more regular diet [67].
When performing EUS-GE, there are various technical approaches that have been well documented in the literature, including antegrade traditional/downstream method, antegrade RV method, retrograde enterogastrostomy, EUS balloon-occluded GE bypass, direct method, and wireless/water-filling technique [68, 69]. A recent retrospective study analyzed the water-filling technique in 107 patients across three European centers with a technical success, clinical success, and AE rate of 94%, 91%, and 10%, respectively [70]. At our center, we place a nasobiliary tube at or just beyond the ligament of Treitz to distend the duodenum using a standard irrigation system as used for luminal endoscopy. After injection of glucagon to paralyze the bowel, we place a 20 mm LAMS with enhanced electrocautery tip using a “freehand” technique. At the moment, there is no method that has proven superior and comparative studies are needed to determine which approach may limit AEs.
As EUS-GE becomes more widely used, we expect this technique to become a more widely used as a therapeutic method that may potentially replace surgery as a first-line option. Indeed, in our practice, we have largely abandoned enteral SEMS in favor of EUS-GE, except in patients with a life-expectancy inferior to 3 months and those with large volume ascites.
EUS-Guided Drainage of Necrotizing Pancreatic Fluid Collections
Therapeutic EUS has also found a role in ESGE guideines for managing complications of acute necrotizing pancreatitis by facilitating transmural drainage necrotic pancreatic fluid collections [71]. Acute pancreatitis is common cause of hospitalization, with annual costs exceeding USD 2 billion, where up to 20% of patient develop severe (necrotizing) pancreatitis [72]. Necrotizing pancreatitis, a feared sequel, is associated with a significant morbidity and mortality - especially when infection is present [73]. Infected and symptomatic fluid collections require multidisciplinary treatment [74]. Drainage and debridement are recommended once the collection encapsulates and matures, which typically takes >4 weeks [75]. With advancements in EUS, there has been a paradigm shift in endoscopically managing these collections, instead of traditional surgical necrosectomy [76]. Percutaneous drainage alone is often avoided whenever possible due to the risk of pancreatocutaneous fistula formation. The last author of this review pioneered early work in this setting, over the past few years EUS drainage of pancreatic fluid collections has evolved to the use of LAMS, with the large diameters (15 and 20 mm) for management of WON. Patients with lower percentages of necrotic debris by volume, those with collections less than 10 cm in size and lack of paracolic extension can often avoid the need for additional interventions such as direct endoscopic necrosectomy [77].
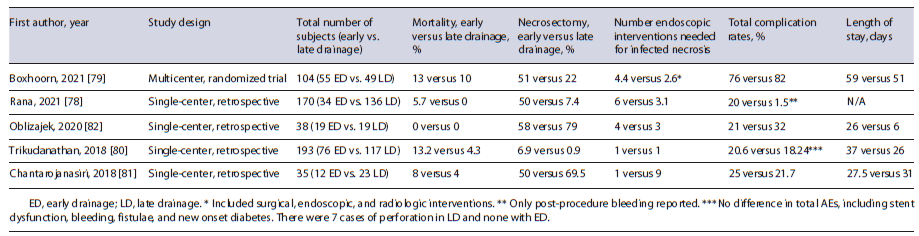
Studies have sought to compare early versus late drainage of infected, necrotic pancreatic collections when clinically indicated (Table 2) [78-82]. Overall these studies reported somewhat similar rates of AEs, though the early drainage groups appeared to require more reinterventions [78-82]. A recent meta-analysis of 6 studies with 630 patients reported no significant differences in technical success, clinical success, mortality, or overall AEs in early (n = 182) versus standard (n = 448) drainage groups [83]. The implementation of LAMS has seemingly revolutionized management by simplifying the technical aspects to potentially limit AEs. Also, the significantly larger stent diameters (15 or 20 mm) can improve drainage and decrease the number of endoscopic sessions needed [84]. A recent study comparing EUS-guided drainage with plastic stents (n = 138) and LAMS (n = 28) found no differences in mortality, complications, or resolution rates but did note LAMS were associated with a shorter time to resolution [78]. Yet, another comparative study between LAMS (n = 78) and traditional cystoenterostomy (n = 78) reported a faster resolution time favoring LAMS (86.9 vs. 133.6 days) [85]. The introduction of a larger, 20 mm LAMS, can further reduce the need for endoscopic necrosectomy [86].
EUS-Guided Ablation of Pancreatic Neoplasms EUS-guided ablation of pancreatic cystic neoplasms has evolved as a reliable minimally invasive option - especially in patients who are poor surgical candidates. With the advent of improved cross-sectional imaging, incidental findings of pancreatic cysts are rising with no overall change in mortality [87]. Pancreatic cystic neoplasms represent a broad spectrum of clinicopathological lesions with varying degrees of malignant potential. Stratifying these lesions based on their malignant potential and presence of symptoms dictates management options i.e. surveillance versus resection [88]. In patients who require treatment, surgical resection is often associated with a high morbidity and mortality; furthermore, some patients may not be ideal operative candidates or decline surgery. It is in this setting where EUS-guided ablation techniques have emerged as an alternative treatment option [89]. Cyst ablation can be performed by injecting ablative agents (ethanol or paclitaxel) or through radiofrequency ablation (RFA). Ablation is indicated for a presumed mucinous cystadenoma or intraductal papillary mucinous neoplasms (IPMNs) that are unilocular or oligolocular, as well as cyst >3 cm or enlarging cyst with a diameter >2 cm [90]. Typically cyst measuring 2-6 cm with fewer than 6 locules respond best to ablation [90, 91]. The cyst is accessed through a transgastric or transduodenal approach where a 22 gauge or 19-gauge fine needle aspiration is used to evacuate the cyst cavity before lavage with the ablative agent takes place [89].
EUS-ablation with ethanol was first used in 2005 as a means to destroy epithelial lining through cell membrane lysis, vascular occlusion and protein denaturation with complete and partial resolution rates ranging from 9% to 78% and 14-40%, respectively [92-95]. This wide range of result was likely influenced by varying study designs, heterogeneity of cyst treated and differing concentrations of ethanol used (80-100%). In addition to varied results, the AEs associated with ethanol ablation, i.e., abdominal pain and acute pancreatitis occur not uncommonly ranging from 3.3% to 33.2% [88].
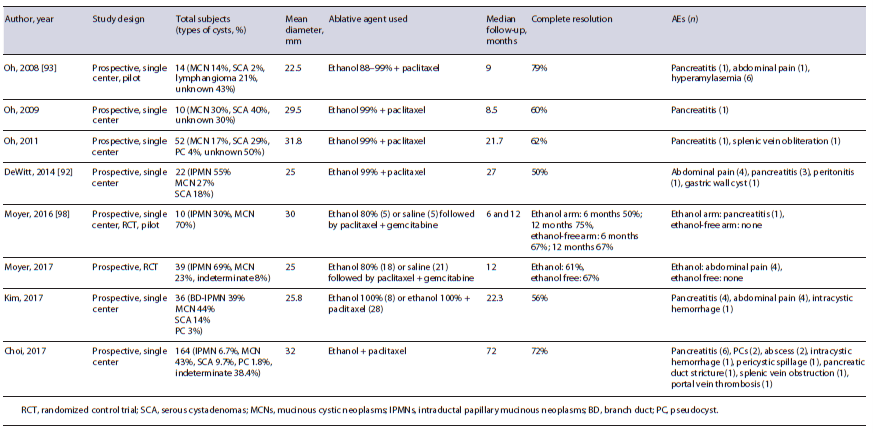
The addition of paclitaxel, a chemotherapeutic agent, has been found to improve complete cyst resolution up to 79% (Table 3) [90]. Paclitaxel was initially used following ethanol lavage in 2008 [93], whereby its hydrophobic and viscus properties were thought to reduce the chances of leaking and providing a longer duration of ablation in the cyst itself through microtubule inhibition [89]. The synergistic effects of ethanol and paclitaxel were promising, and one study found that post-ablation neoplastic DNA mutations were disrupted and eliminated in 72% of cases [92]. Compared to ethanol ablation alone, post-ablation AEs (15 vs. 21.7%) and complete resolution rates (63.6 vs. 32.8%) are significantly improved using paclitaxel-based regimens [96]. There was a concern that post-procedural acute pancreatitis (3.3-9.8%) was associated with ethanol extravasation into the pancreatic parenchyma [88]. In an effort to determine the safety and efficacy of alcohol-free ablation, a prospective double-blind randomized trial (known as the CHARM trial) compared an admixture of paclitaxel and gemcitabine with or without 80% ethanol in a cohort of 39 patients with mucinous-type cysts [97]. The investigators found that there was no major difference in complete resolution rates (61% with and 67% without ethanol) with an added benefit of no AEs experienced in the alcohol-free cohort. In order to validate these findings on larger scale, the CHARM II trial is currently underway with an expected study completion date in April 2023 [98].
Alternatively, RFA can be performed through electromagnetic energy and high-frequency alternating currents via mono- or bipolar probe, using an echogenic 19-gauge needle tip, that can induce cell death by causing coagulative necrosis, hyperthermic injury, and a delayed immune response to the cyst in question [88, 89]. When this energy is transmitted to the targeted lesion, echogenic bubbles can be visualized on EUS. Only a handful of studies have explored RFA as means to treat pancreatic cysts and pancreatic neuroendocrine tumors (pNETS) with prom-ising results [99-101]. A recent meta-analysis found that location of a pNET in the pancreatic head/neck was a positive predictor of clinical success [102]. The meta regression reported a pooled clinical success and AE rate of 85.2% and 14.1%, respectively [102]. A prospective multicenter study including 16 IPMNs, 14 pNETs, and 1 mucinous cyst adenoma reported a very low AE rate (only two events occurring in the first 2 patients treated), which was virtually eliminated when prophylactic measures (i.e., antibiotics, rectal diclofenac and cyst aspiration before RFA) were taken [99]. Of note, in regards to pNETs, the best results appear to be for treatment of insulinomas and non-functional pNETs ≤2 cm.
Still in its infancy, EUS-guided ablation may prove useful in selected patients with high risk or symptomatic pancreatic cysts, further studies will be needed to determine if there is a cost saving and/or mortality reducing component. In patients who are not surgical candidates, it is possible that EUS-guided ablation could alter surveillance recommendations moving forward, though comparative studies are needed. In current guidelines, EUS-guided cyst ablation should not be performed outside a dedicated investigation protocol.
EUS-Guided Coil Embolization
While esophageal varices are more common, GVs are associated with severe bleeding, higher mortality rates, and rebleeding episodes [103]. The therapeutic endoscopic armamentarium for GV is somewhat limited, though in recent years EUS methods have found a role in managing these serious bleeds. Injecting cyanoacrylate glue has traditionally been used to resolve acute bleeding and provide secondary prophylaxis. Yet, bleeding is influenced by the size and wall tension of the varix, and endoscopic injection of glue does not always allow for full visualization, which can increase the risks of rebleeding. Furthermore, this technique is technically challenging and associated with severe AEs, including systemic embolization [104]. Other disadvantages include inadvertent unroofing of the varix, deep ulcerations at the injection site, and damage to the endoscope itself [105].
In this context, EUS-guided injection provides unique luminal views that can fully characterize the varix and confirm obliteration on doppler ultrasound while reducing the risks of glue embolization (Fig. 3) [106]. Utilizing this approach, EUS-guided coil embolization has been investigated as an additional hemostatic method that promotes clot formation [104]. In a large study of 152 patients, combination therapy with cyanoacrylate glue and coils was technically successful in 99% of cases with only three episodes of post-treatment bleeding [107]. The coils serve as a scaffold with synthetic fiber that contains and minimizes the amount of glue needed [108]. Compared to glue injection alone, combination therapy requires fewer endoscopic sessions while limiting AEs [109]. A recent randomized trial compared combination (cyanoacrylate plus coil) therapy (n = 30) to coil monotherapy (n = 30) and found that combination therapy led to significantly higher rates of obliteration (86.7 vs. 13.3%) with lower rates of rebleeding or reintervention needed [110].
In an effort to further describe the benefits of combination therapy, a recent meta-analysis of 11 studies with 536 patients, confirmed that combination therapy resulted in higher rates of technical and clinical success com-pared to cyanoacrylate alone [111]. In terms of AEs, combination therapy and coil monotherapy demonstrated comparable results (10 vs. 3%), while cyanoacrylate injection was associated with a 21% adverse even rate [111]. Interestingly, recent studies have postulated the replacement of glue with an absorbable gelatin sponge (AGS), which is typically used as a hemostatic agent in interventional radiology and surgical procedures [105, 112, 113]. The AGS is a purified water-insoluble plug that can absorb 45 times its volume in blood [105, 112, 113]. An added benefit is that it is not associated with post-treatment ulceration and cannot damage the endoscope. In a matched cohort study, the use of coil embolization plus AGS was superior to glue injection alone in terms of lower rebleeding rates, transfusion requirements and rates with up to 9 months of follow-up [112]. The authors added that in their cohort they used more coils (∼8 per case)
compared to 1-3 coils used in a prior study [114]. Their thought process was that using a significantly larger coil volume could aggressively obliterate feeder vessels at multiple vascular points [112]. While this technique is promising, AGS is not FDA approved and is therefore limited in use.
Another alternative is the use of thrombin injection, which can achieve hemostasis by converting fibrinogen to fibrin thereby promoting clot production and platelet aggregation [115]. One study by Frost and Hebbar [115] demonstrated the feasibility and efficacy of this approach using an EUS-guided injection technique. The authors treated 5 patients for primary prophylaxis and three with active bleeding using EUS-thrombin - and found that only 1 patient with active bleeding failed to achieve hemostasis [115]. There were no AEs. Another randomized control trial (RCT) by Lo et al. [116] compared endoscopic thrombin (n = 33) to cyanoacrylate (n = 35) and found that both groups had similar rates of hemostasis, but the thrombin cohort experienced lower rates of AEs (12 vs. 51%) with no instances of gastric ulceration. Other added benefits of thrombin are the excellent safety profile (minimal risk of embolism or ulceration compared to glue injection) and ease of use. Additional EUS-guided studies are needed to determine its role in variceal hemorrhage.
The rapid adaptation of EUS-guided coil embolization is emerging as a promising treatment option for GV. The ability to decrease complications while maintain effective hemostasis should reduce the costs associated with GV bleeding, though further studies will be needed.
EUS-Guided Gallbladder Drainage for Acute Cholecystitis
EUS-guided gallbladder drainage (EUS-GBD) has also emerged as novel and clinical useful management option in patients with symptomatic cholelithiasis and/ or acute cholecystitis who are not optimal surgical candidates. In instances of acute cholecystitis, early laparoscopic cholecystectomy remains the gold standard [117]. However, patients with advanced age, poor performance status, significant comorbidities, or prior abdominal surgery causing adhesions may be unfit for surgery due to high rates of morbidity and mortality [118]. A delay in surgery can increase the risk of gallstone-related complications by 14% at 6 weeks, 19% at 12 weeks, and 29% at 1 year [119]. Thus, providing alternative routes of decompression via a percutaneous or endoscopic approach have been investigated. Traditionally, a percutaneous cholecystostomy has been performed, though tube maintenance, dysfunction and patient discomfort are often challenging for patients [118]. A percutaneous approach may worsen a patient’s quality of life, while also increasing costs associated with long-term care issues related to readmissions and reinterventions, with AEs ranging from 4% to 51% [120].
Since its first description in 2007 by Baron and Topazian, EUS-GBD has rapidly evolved with improved clinical outcomes following the introduction of LAMS [121]. Over time, the use of plastic stents, SEMS, and then LAMS has led to ongoing technical and clinical success with a dramatic reduction in AEs (18.2% plastic stent, 12.3% SEMS, 9.9% LAMS) [122]. When compared to percutaneous drainage, EUS-GBD serves an opportunity to treat poor surgical candidates through a minimally invasive approach that lowers rates of reintervention and unplanned readmissions [120, 123-126]. Two recent comparative meta-analyses found no difference in technical or clinical success; however, they demonstrated that EUS-GBD was associated with lower AEs, shorter hospital stays, and fewer reinterventions which lead to decreased readmissions [120, 124]. Similar findings were seen in a randomized control trial of 80 patients undergoing EUS or percutaneous gallbladder drainage in high risk surgical candidates [123]. It has also been associated with significantly lower post-procedural pain [126].
When compared to the gold standard (laparoscopic cholecystectomy), a propensity score analysis found that EUS-GBD was comparable (technical success 100 vs. 100%, clinical success 93.3 vs. 100%, 30 day AEs 13.3 vs. 10%) - suggesting this method can be considered as a reliable alternative in patients who are not ideal operative candidates [127]. It has also been studied in 15 patients with cirrhosis (average MELD 15 ± 7) with a technical success rate of 93.3% and two AEs (1 mild, 1 severe) [128].
With increasing use of EUS-GBD with LAMS, higher risk patients are being treated. It is important to comment that with a permanent fistula created with LAMS a bridge to laparoscopic cholecystectomy may not be possible [118]. Though successful surgery has been documented in patients stented with plastic stents [129]. Future studies may yet demonstrate the feasibility of safe laparoscopic resection after LAMS placement, perhaps after resolution of inflammatory changes, endoscopic removal of LAMS, and fistula closure.
Conclusion
Over the past 20-years therapeutic, EUS has catapulted itself as reliable therapeutic tool that has expanded the field of interventional gastroenterology. Translating theoretical implications into practical methods has allowed EUS-guided therapies to change practice management worldwide. We believe it is inevitable that EUS-guided transmural biliary drainage will be accepted as an alternative to ERCP for the relief of malignant biliary obstruction. Similarly, EUS-guided GE will also become the accepted treatment for relief of malignant GOO over S-GJ and endoscopic luminal stent placement. Yet, at the present time, a lack of standardized training and limited ex-pertise will confine these techniques to high volume centers where multidisciplinary ancillary support is required.