Introduction
Over the last few years, therapeutic endoscopy has evolved into the preferred approach, or at least a valid alternative, for management of several gastrointestinal (GI) conditions, wherein surgery was considered the standard therapy for decades [1]. Endoscopic stenting is one such aspect of therapeutic endoscopy that has witnessed noteworthy advancements. Traditionally, the main indications for endoscopic stenting were limited to the palliation of malignant disorders, such as obstructive esophageal cancer, malignant gastric outlet obstruction (GOO), and malignant colonic obstruction [2]. More recently, indications for endoscopic stenting have gradually expanded to include a variety of nonmalignant/non-obstructive disorders, such as external compression of the GI tract, GI transmural defects (e.g., perforations, fistulae, and leaks), and selected cases of refractory benign strictures [3]. Moreover, advances in biotechnology and clinical expertise have helped mitigate stent-related adverse events (AEs) [4]. In this review, we aim to summarize the evidence and experience supporting the best practices in luminal endoscopic stenting, with a specific focus on esophageal, gastroduodenal, and colonic stenting (Table 1).
Esophageal Stenting
Malignant Esophageal Cancer
Palliation of Malignant Dysphagia
The main goal of esophageal stenting is palliation of malignant dysphagia in patients with esophageal cancer to improve nutritional intake. Although stenting provides a rapid relief of dysphagia symptoms, it is preferable in patients with an expected short survival (<3 months) (Fig. 1) [5]. A meta-analysis by Wang et al. included three randomized clinical trials (RCTs) and showed similar outcomes between fully covered self-expandable metal stent (FC-SEMS) and partially covered SEMS (PC-SEMS), without differences in stent migration, obstruction, or bleeding [6]. Pooled data from available studies showed a major AE rate of 18% with PC-SEMS and 21% with FC-SEMS (most frequently reflux, severe pain, bleeding, and ingrowth/overgrowth) [7]. Different stent designs have been developed in order to prolong stent patency and reduce AEs; however, this is hard to accomplish as stents do not affect natural history of the disease. Regarding anti-reflux stents, for example, a 2019 meta-analysis [8] and a subsequent RCT [9] failed to prove their superiority regarding improvement of reflux, dysphagia score, or related AEs (stent migration, bleeding, and obstruction).

Fig. 1 Patient with dysphagia secondary to an esophageal squamous cell carcinoma located in the mid esophagus. a Endoscopic image showing proximal view of the lesion. b, c Fluoroscopic and endoscopic images after placement of a partially covered 150 × 20 mm self-expandable metal stent.
One major drawback of stent use in patients with longer survival is the increased risk of stent dysfunction and AE occurrence. Even though SEMSs are associated with earlier symptom relief, for patients with longer expected survival (≥3 months), brachytherapy seems to provide better quality of life, long-term dysphagia relief, and fewer AEs, when compared to SEMS placement [10, 11]. However, despite being associated with better long-term results, brachytherapy is underused in clinical practice [12, 13]. Overall severe AEs from brachytherapy alone may occur in up to 23% of cases, mostly including brachy-therapy-related stenosis (12%) and fistula formation (8%) [14]. The effect of combined brachytherapy and stenting on AE rates is not completely clear; however, it seems to provide better dysphagia relief in patients with survival longer than 3 months and higher overall survival, compared to SEMS alone [15]. Patients may also be palliated with external beam radiotherapy (EBRT) alone [16, 17]. A recent propensity score-matched analysis that compared EBRT alone with brachytherapy alone suggested that EBRT may offer a faster and safer dysphagia relief compared to brachytherapy, with similar long-term outcomes [18]. A recent RCT that compared EBRT alone with a combination of EBRT and chemotherapy found that EBRT alone had similar dysphagia relief and survival as the combination therapy, but fewer AEs [19].
Some retrospective cohorts evaluated patients submitted to SEMS placement, with ≥6-month survival, and concluded that SEMS may be a valid alternative, especially in centers where brachytherapy is not widely available. Despite the increased risk of AEs over time, most of them can be managed endoscopically [20-22].
Irradiation stents have been developed to combine advantages of both SEMS and radiotherapy. A 2017 [23] and a 2021 [24] meta-analysis comparing irradiation SEMS (loaded with 125I beads) versus traditional SEMS showed prolonged patient survival and stent patency with irradiation stents, with no differences in AE rates. Biodegradable stent (BDS) role in the palliation of malignant dysphagia is not adequately defined and should not yet be considered a valid alternative to SEMS [25].
Recommendation: Patients with life expectancy of less than 3 months or suffering from severe dysphagia should be considered for SEMS placement. FC- or PC-SEMS may be considered. Brachytherapy should be considered when available in patients with expected longer survival.
Bridge-To-Surgery Patients
In the curative setting, as bridge to surgery, SEMS placement is not recommended by most recent guidelines, since it may be associated with worse oncologic outcomes, a lower rate of R0 resection, increased 3-year follow-up recurrence, lower overall survival, and a higher rate of major AEs [26, 27]. Although some recent studies reported no differences in R0 resection rate and overall survival, SEMS placement may increase postoperative morbidity and mean operative time making surgery more challenging [28-30]. Nevertheless, esophageal stents are helpful to ameliorate nutritional status during or before neoadjuvant therapy and/or surgery [31]. Only two studies addressed the potential advantages of esophageal stents compared to standard feeding techniques, with SEMS being associated with lower rates of chemoradiotherapy interruption, greater improvement of albumin, lower body weight loss, and major operative complications, when compared to feeding tube or oral nutrition [32], while SEPSs were considered at least as safe and effective as surgical jejunostomy (no differences in weight loss and albumin) [33]. Available studies lack information about stent dwell time till surgery [29, 31, 34, 35]. However, a study reported no differences between SEMS and non-SEMS groups in median time from diagnosis-to-surgery (132 vs. 140 days, p = 1.0) [30].
Recommendation: Currently, SEMSs are not recommended in the curative setting, as bridge to surgery.
Esophago-Respiratory Fistulas
When a fistula develops between the esophagus and trachea or bronchi, the underlying malignancy is invariably incurable, regardless of the primary site. This condition is associated with a poor survival, so palliative management is preferred in most cases [36]. Esophageal stents may be used for treatment of malignant tracheo- and bronchoesophageal fistulas, due to their safety and effectiveness profile, with lower morbidity and mortality compared to surgery [37]. The reported clinical success of SEMS ranges from 67 to 100%, and reintervention is needed in up to 39% of the cases, mainly due to stent migration, persistent fistula, and aspiration [38].
Combined placement of stents in both the esophagus and the tracheobronchial tree is another management strategy for esophago-respiratory fistulas (ERF), being indicated if esophageal stenting could compromise the respiratory tract via extrinsic compression (more likely in mid-/proximal ERF); if there is a pre-existing tracheal stenosis; and in cases of large fistulas (>20 mm) [39-41]. However, patients who require dual esophageal and airway stenting are at risk for fistula worsening due to pressure necrosis on both sides of the fistula from the two opposing stents [42]. Broncho-esophageal fistulas are reported in 5-10% of patients with esophageal cancer; in most of these cases, placement of a single stent, either a tracheobronchial or an esophageal stent, is enough to seal the fistula [43].
If double stenting is performed, airway stenting should be placed first to reduce the risk of airway compromise and the risk of esophageal stent migration [44]. Mean survival does not seem to be impacted by single or double stenting [45].
Recommendation: FC-SEMS or PC-SEMS can be considered for the treatment of ERF, as long as the fistula is covered by the stent membrane. Double stenting should be considered if risk of respiratory tract compromise secondary to the esophageal SEMS, if pre-existing tracheal stenosis and if large fistulas (>20 mm).
Benign Disorders
Refractory Benign Esophageal Strictures
Esophageal stents have been studied as an option for refractory benign esophageal strictures (RBES). They should only be considered after therapeutic failure of other endoscopic alternatives, like dilation or incisional therapy. A 2015 meta-analysis from Fuccio et al. [46] (n = 444) reported a clinical success of 40.5% and an overall AE rate of 20.6%, with stent migration being the most common AE (28.6%). To prevent stent migration, a variety of techniques and devices have been used with FC-SEMS, such as through-the-scope clips [47], over-the-scope clips (OTSC) [48], and endoscopic suturing [49]. Different retrospective single-center and multicenter studies [49-51] and a meta-analysis [52] support the supposition that endoscopic stent fixation in benign esophageal stenting prevents stent migration. Only one study compared different stent fixation techniques, with OTSC significantly decreasing stent migration rates as compared to no fixation or endoscopic suturing, while also increasing clinical success rate [53]. Two studies found that previous stent migration was a risk factor for similar future events; therefore, stent fixation should be considered in patients with high risk for stent dislocation and/or previous stent migration.
A stent dwell time of 6-12 weeks is recommended, to allow stricture remodeling and at the same time prevent stent embedment [26]. FC-SEMSs are preferable over PC-SEMS for RBES treatment, since PC-SEMSs are associated with stent embedment, leading to an increased risk of AEs during stent removal [54]. Despite different available methods for embedded PC-SEMS removal (stent-in-stent [SIS], argon plasma coagulation, overtube technique, inversion technique), comparative studies for these different techniques are lacking. Overtube and inversion techniques employ shear forces on a distinct area to facilitate stent extraction; however, these techniques may be more invasive and potentially lead to perforation. Argon plasma coagulation technique, by using heat for removal, is less complicated but could potentially fail in severe cases. SIS technique (placement of FC-SEMS over-lapping the embedded PC-SEMS, followed by removal of both after 10-14 days) is more expensive and time-consuming, but it is the best-studied procedure and is usually recommended because of the lowest expected complication rate [55].
A meta-analysis of 18 studies did not show significant differences in clinical success, stent migration, and complication rates between BDS, SEMS, or SEPS [46]. However, patients with BDS (Fig. 2) may require fewer endoscopic reinterventions [56-58]. Despite this, updated European Society of Gastrointestinal Endoscopy (ESGE) guidelines do not recommend BDS over other stents [26]. Lumen-apposing metal stents (LAMSs) also have been evaluated for RBES, but available data are limited to small case series [59-62]. They may be considered in patients with short RBES up to 10 mm (Fig. 3). In patients with persistent dysphagia despite stent placement, surgery should be considered. Self-dilatation with boogies may be an option for poor surgical candidates [63].
Recommendation: Temporary placement of self-expandable stents may be considered for RBES. No recommendation can be made regarding a specific type of ex-pandable stent. When SEMSs are used, FC-SEMS should be preferred. Stent fixation techniques can be used to mitigate migration risk.

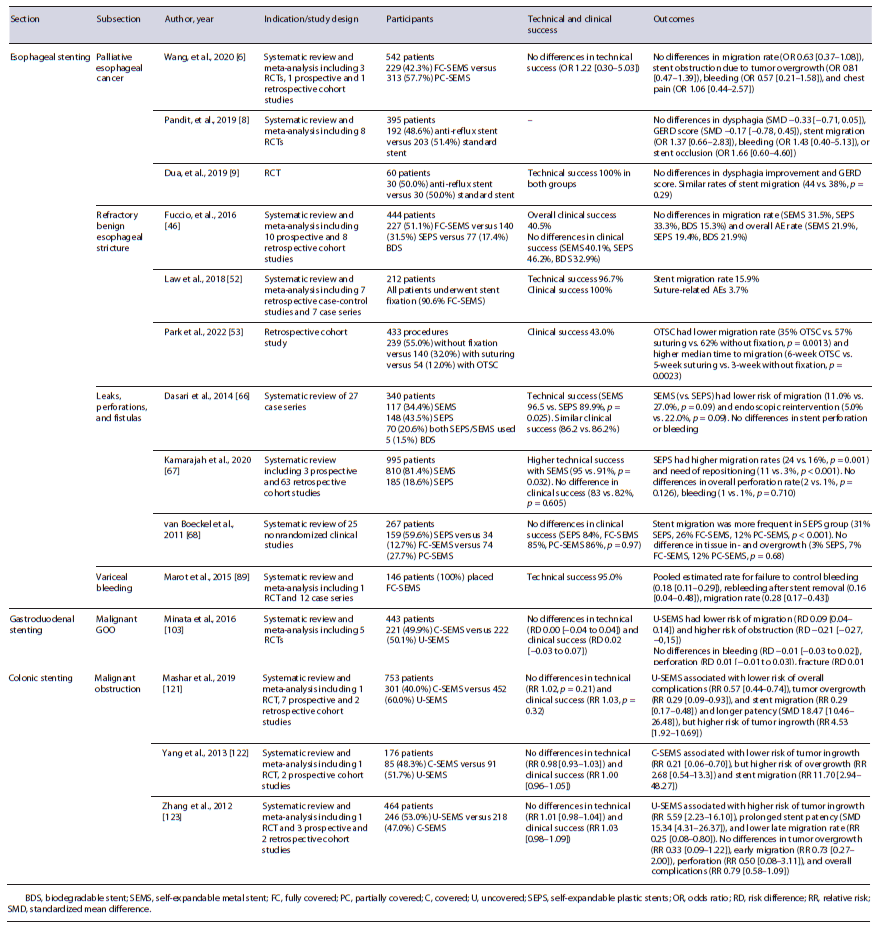
Fig. 2 Patient with a refractory benign esophageal stricture due to caustic ingestion, submitted to multiple endoscopic treatments (Savary and balloon dilatation, fully covered self-expandable metal stent placement). a Endoscopic image of esophageal stricture. b Fluoroscopic image revealing a 2-cm-long stricture after contrast instillation. c-f Endoscopic and fluoroscopic images after placement of a 25/20/25 × 100-mm biodegradable noncovered stent.
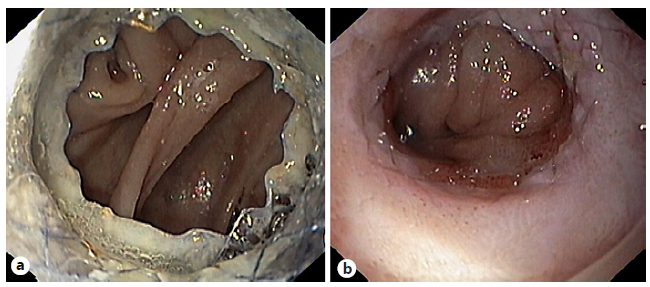
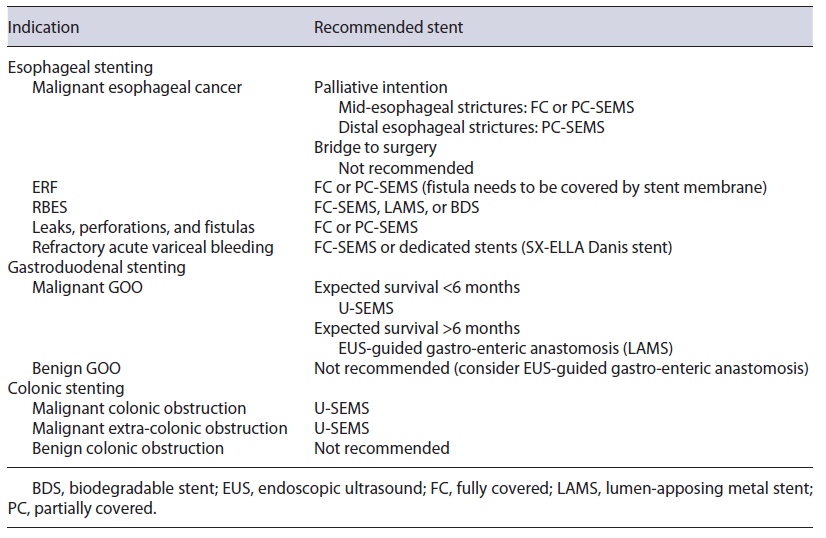
Fig. 3 Patient with a refractory esophago-jejunal anastomotic stricture who under-went placement of a lumen-apposing metal stent (LAMS) across the stricture. a Endoscopic image of the LAMS placed across the stricture. b Esophagojejunal anastomotic stricture remodeling after LAMS removal.
Leaks, Perforations, and Fistulas
Recent advances in endoscopy have prompted a paradigm shift in the management of esophageal leaks, perforations, and fistulas, from surgery to minimally invasive endoscopic approaches [64]. Even though these terms are often used interchangeably, in strict terms, they are completely different [65]. Therefore, their treatment should be individualized.
Based on three systematic reviews on the use of PC-SEMS, FC-SEMS, and SEPS in anastomotic leaks and perforations, the clinical success rate of esophageal stent placement is 81-87%, with no difference among the stent types [66-68]. Only two studies [69, 70] evaluated fistulas individually, with clinical success ranging from 45.5 to 90.1%; however, SEMSs were used almost always in combination with other endoscopic/pulmonary techniques; clinical success decreased with orifice size increase [69]. Huh et al. [71] and Suzuki et al. [72] reported higher clinical success for perforations compared to leaks (100% vs. 60-80%), with anastomotic leak group needing a longer stent dwell time (≥4 weeks) compared with the perforation group (75% vs. 27.3%, p = 0.022). Overall AEs ranged from 3.8 to 50% [70, 71, 73, 74], with stent migration (8.5-42%) [67, 74-78] and strictures or stentinduced ulcers (3-48%) [73, 79] being the commonest. Even though stent-related AEs are typically managed endoscopically, severe AEs (14.7%) [80] can occur, requiring nonendoscopic advanced management.
The selection of the right stent design also remains a challenge (Fig. 4). Even though clinical success rates are comparable, SEMSs perform better than SEPS in leaks and perforations, with higher technical success (95% vs. 91%, p = 0.032), reduced risk of migration (16% vs. 24%, p = 0.001), and need for stent repositioning (3% vs. 11%, p < 0.001), as well as lower risk of perforation when considering anastomotic leaks only (0% vs. 2%, p = 0.013) [67]. Migration rates are higher with FC-SEMS versus PC-SEMS (odds ratio [OR] 2.44, 95% CI 1.13-5.31; p = 0.024) [77]; however, suturing FC-SEMS may render migration rates similar to PC-SEMS (adjusted OR 0.56, 95% CI 0.15-2.00; p = 0.37), without the difficulties in removal of PC-SEMS and a lower risk of AEs (21% vs. 46%, p = 0.37) [51]. Shim technique (silk thread attached to proximal end of the stent and to the patient ear via the nares) [81] as well as stents with wider diameters [77, 82] may also result in lower migration rates. Data regarding the role of BDS in management of esophageal transmural defects are limited. Only two studies, comprising 13 and 4 patients, are available: despite a clinical success of 77.8-100%, mucosal reaction (2/4 patients) is a drawback, causing dysphagia and requiring endoscopic dilation [83, 84].
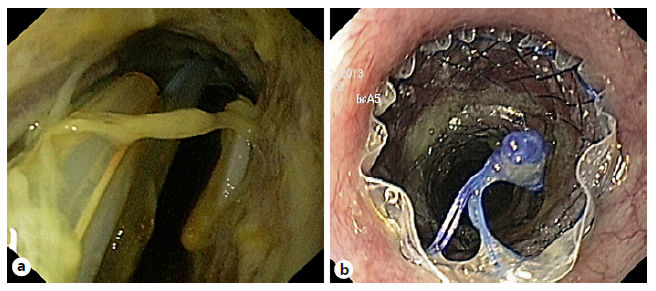
Fig. 4 Patient with an anastomotic leak after total gastrectomy. a Endoscopic image showing an anastomotic leak occupying more than 50% of the luminal circumference. b Immediately after placement of a fully covered self-expandable metal stent.
Predictive factors for stent failure/mortality include persistence of fistula orifice after 6 months of endoscopic treatment (OR 44, 95% CI 3.38-573.4; p = 0.004) [69], larger fistula size [69], if stent was used after failure of revisional therapy compared with stent used as initial treatment (55% vs. 100%, p = 0.013) [73], continuous leakage after stent placement [85], decreased physical performance preoperatively [85], and concomitant esophagotracheal fistula [85]. Van Halsema et al. [86] developed a prediction rule for successful stent placement in the context of benign upper GI leakage, consisting of etiology, location, size of the leak, and C-reactive protein level at diagnosis. Iatrogenic/spontaneous perforation (vs. leaks or fistulas), proximal defect location (<25 cm from the incisive), lower C-reactive protein levels, and smaller defect sizes (<1 cm) were considered predictors of better outcomes; after validation in a different patient cohort, the rule was found to significantly discriminate between failure (NPV 86%) and success (PPV 87%) of stent placement in patients with a predicted low (≤50%) or high (≥70%) clinical success, respectively.
ESGE-updated guidelines recommend removing the stent 6-8 weeks after placement [26], even though there is a tendency to remove or replace stents at shorter interval times, to reduce stent-related AEs. SEMSs have been compared to endoscopic vacuum therapy for the treatment of post-surgical leaks in two systematic reviews and meta-analyses [87, 88], with endoscopic vacuum therapy being associated with higher leak closure, more endoscopic device changes, shorter duration of treatment, and lower rates of mortality and/or major complications. Given the high complexity and particularities of transmural defects, in most cases a multimodality approach is adopted, but endoscopic stenting remains one of the most frequently used options in these patients [77].
Recommendation: Temporary SEMS placement can be considered for leaks, perforations, and fistulae. Considering the complexity of these transmural defects, a multi-modality approach is often preferred.
Acute Variceal Bleeding
In the setting of refractory acute variceal bleeding, several systematic reviews and meta-analyses [89-91] support use of SEMS in successful control of severe or refractory acute variceal bleeding, without significant device-related AEs (Fig. 5). This strategy is often used as a bridge to transjugular intrahepatic portosystemic shunt or liver transplantation in a significant proportion of patients [89], and 6-week survival is mostly related to the severity of the underlying liver disease. Dedicated FC-SEMSs (SX-Ella Danis) for esophageal variceal bleeding are available; when used, retrieval should be performed using a specifically designed system [92, 93]. There is only one RCT comparing FC-SEMS (SX-Ella Danis stent) with balloon tamponade [94], with successful therapy more frequent in the stent group (66% vs. 20%), with a significantly higher rate for control of bleeding (85% vs. 47%), lower transfusion requirements, and a lower incidence of seri-ous AEs (15% vs. 47%), mainly due to differences in aspiration pneumonia (0 vs. 5) and esophageal tear (1 patient in the balloon tamponade group); no significant difference in 6-week survival was observed (54% vs. 40%). In most published studies, FC-SEMSs were left in place for up to 2 weeks [95-98], although extended dwell time up to 30 days has been reported. Recommendation: FC-SEMS placement may be considered for the treatment of severe or refractory esophageal variceal bleeding, as a bridge to transjugular intrahepatic portosystemic shunt or liver transplantation.
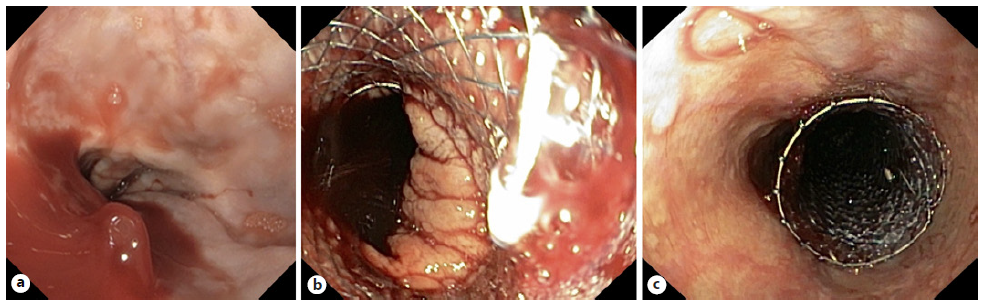
Fig. 5 Patient with cirrhosis Child-Pugh C and severe esophageal variceal bleeding. a Endoscopic image showing active bleeding from an esophageal varix. b, c Endoscopic image after placement of a dedicated fully covered self-expandable metal stent (SX-Ella Danis stent) with bleeding control.
Gastroduodenal Stenting
GOO typically involves the distal stomach and/or the proximal small bowel (although it may also affect the distal small bowel) and may be secondary to mechanical/obstructive or motility causes. Mechanical obstructions can be benign or malignant [99]. The traditional approach for management of malignant GOO involves surgical gastrojejunostomy, via either open or laparoscopic access, although less invasive alternatives including endoscopic placement of luminal SEMS (Fig. 6) and, more recently, endoscopic ultrasound (EUS)-guided gastroenterostomies have become increasingly popular. On the contrary, benign GOO is generally managed with endoscopic balloon dilation (EBD), reserving more invasive techniques for EBD refractory cases [100].
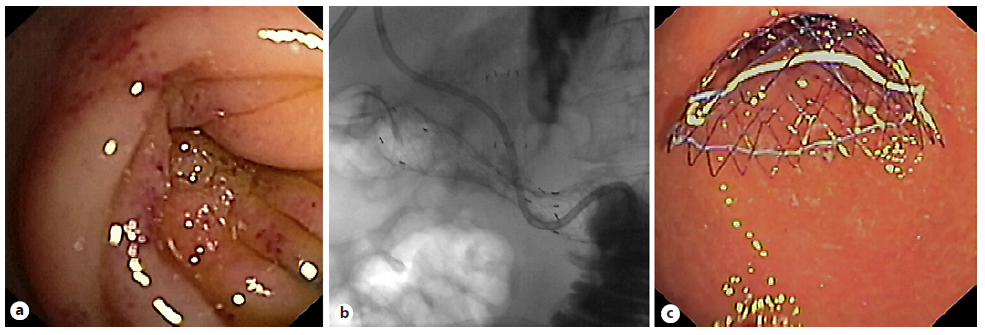
Fig. 6 a Patient with pancreatic cancer and previous biliary self-expandable metal stent with gastric outlet ob-struction due to tumor invasion in the second portion of the duodenum. b, c Fluoroscopic and endoscopic images after placement of an uncovered 140 × 20 mm self-expandable metal stent.
A recent meta-analysis favored surgical gastrojejunostomy over SEMS due to longer overall survival and fewer needs for reintervention. Although postoperative mortality and AEs were similar between the two groups, the SEMS group had shorter hospital stay and shorter time to resume oral intake [101]. Technical and clinical success was 83.3-100% and 75-100%, respectively [101]. Therefore, patients with short life expectancy (<6 months), especially those who are high surgical risk, may be better candidates for luminal SEMS [102]. Regarding the stent type for GOO, a 2016 meta-analysis noted no significant difference in technical or clinical success, AEs, and reintervention for covered SEMS (C-SEMS) and uncovered SEMS (U-SEMS), but as expected, migration rate was higher, while obstruction rate was lower with C-SEMS [103].
In patients with combined malignant duodenal and biliary obstruction, “double stenting” should be the standard of care practice, due to its lower invasiveness and shorter recovery time [104]. Regarding approach for biliary stenting, endoscopic retrograde cholangiopancreatography stenting might be associated with a lower AE rate compared to EUS-guided biliary drainage and should be considered the preferred approach, when feasible [105].
Only a few case series have been published regarding SEMS as salvage therapy for benign GOO who failed initial EBD attempt. Despite symptomatic improvement in almost 80% of the patients, SEMS placement is limited by stent migration rates up to 47% [106, 107], with no robust evidence to recommend SEMS over surgery in these patients [102].
Recommendation: In patients with life expectancy below 6 months, especially if at high surgical risk, luminal SEMS can be considered. Otherwise, gastroenteric anastomosis should be considered, either surgical or endoscopic. Combined malignant duodenal and biliary obstruction should be approached with “double stenting.”
Colonic Stenting
Malignant Colonic Obstruction
Colonic stenting is a valid alternative to emergency surgery in patients with malignant colonic obstruction, either as bridge to surgery or palliative intention. Prophylactic stenting, in the absence of symptomatic obstruction, should not be performed. Most of the literature concerns left-sided obstructing colon cancer (Fig. 7), excluding (distal) rectal cancers; however, SEMS may also be successfully placed in malignant obstruction of the proximal/right colon [108]. As a bridge to surgery, colonic stenting is associated with fewer overall AEs, similar 30-day mortality rate, and a higher proportion of primary anastomoses, compared to emergency surgery. Even though pre-surgical colon stenting (as bridge therapy) may be associated with a higher overall tumor recurrence, this did not translate into a significant difference in terms of disease-free survival or overall survival on 3- and 5-year follow-up [109, 110]. The worse oncologic outcomes seem to be explained by stent-related perforations, with overall survival being better in studies with lower perforation rates. The ideal time interval for surgery after colonic stenting should be balanced between stent-related AEs (reduced by a short interval) and surgical outcomes (improved by a longer interval). ESGE-updated guideline suggests a 2-week interval between stent placement and surgery [111]. In patients who are not good candidates for colonic stenting (locally advanced disease requiring neo-adjuvant therapy or longer stenosis) or who fail stent placement, a decompressing stoma may be an alternative as a bridge to surgery, allowing a higher chance of successful primary anastomosis [112].
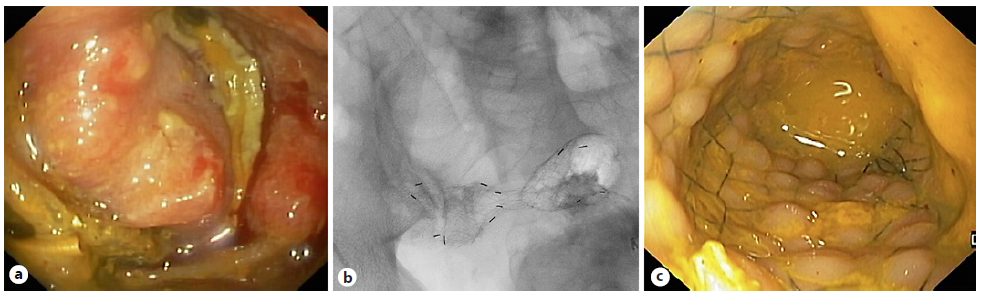
Fig. 7 Patient with malignant colonic obstruction due to colorectal cancer. a, b Fluoroscopic and endoscopic images after placement of an uncovered 80 × 20 mm self-expandable metal stent.
In a palliative setting, most meta-analyses have demonstrated SEMS to be associated with lower short-term mortality, hospital stay, early AEs, stoma rates, and time to initiation of chemotherapy, compared to emergency surgery. Conversely, late AEs were more frequent in the SEMS group [113-117]. Chemotherapy does not seem to be a risk factor for colonic stent-related complications in general; however, in patients already receiving bevacizumab, stent placement is not advised due to high risk of perforation [111].
Extra-colonic malignancy complicated with colonic obstruction may benefit from palliative colonic stenting and is associated with fewer AEs compared to decompressive surgery. Unfortunately, technical and clinical success rates are lower compared to primary colonic cancer [118-120].
In terms of type of colonic stents, 3 meta-analyses have compared U-SEMS and C-SEMS, noting similar technical and clinical success, but U-SEMSs were associated with fewer overall AEs, including less tumor overgrowth, lower migration rates, longer patency, and fewer reinsertions, although at the cost of higher risk of tumor in-growth [121-123]. The main complications included perforation, stent failure, stent migration, and stent re-obstruction [124]. Migration should be treated with stent replacement or SIS technique in the palliative setting, and early surgery in the bridge-to-surgery patients [125].
Recommendation: SEMS can be considered for malignant colonic obstruction treatment as bridge to surgery (advantages and disadvantages of its placement must be discussed with the patient) or in palliative setting. Despite lower success rate, SEMS can also be used for extra-colonic malignant obstruction treatment.
Benign Colonic Obstruction
In recent years, the use of SEMS has been extended to treatment of benign GI strictures secondary to diverticulitis, radiation colitis, inflammatory bowel disease, and endometriosis, as well to management of post-anastomotic colonic leaks, strictures, and fistulas [126]. However, majority of data available in this regard are derived from retrospective studies.
Several studies have reported outcomes of colonic stenting for diverticulitis-associated strictures, as a bridge to surgery or for palliation (in poor surgical candidates). A systematic review (n = 66) concluded that the AE rate was not acceptable to warrant its use (11/66 perforations) [127]. Regarding fibrostenosic Crohn’s disease (CD) refractory to medical treatment, SEMS use is only described in small case series [128]. The largest case series, with a stent dwell time of 4 weeks, showed treatment efficacy of 64.7%, with one AE (proximal stent migration). Distal stent migration (52%) was not considered an AE but rather an incident [129]. A systematic review evaluated SEMS placement for the management of colorectal surgical complications including anastomotic strictures, leaks, or fistulas. A high early success rate (73.3%) was observed; however, anastomotic strictures were more challenging to treat, as around 50% of the patients had persistent stenosis and 26% required EBD after stent placement [130]. Complications were reported in 41.5% patients, mainly SEMS migration, explained by the inherent characteristics of C-SEMS [131]. Colonic stent placement in bowel obstruction due to endo-metriosis, colonic fistulas, radiation-induced stenosis, or ischemic colitis is also reported in literature, but only as case reports or short case series [126, 127].
The largest case series of BDS in colon and ileocolic anastomotic strictures report a technical success of 90-100% but only a modest stricture resolution of 45-83%. Unlike in esophageal strictures, mucosal hyperplastic reaction after BDS placement has not been reported in intestinal strictures [132, 133]. Use of BDS for CD strictures can theoretically overcome the shortcomings of SEMS (stent migration and need for stent removal); however, absence of biodegradable through-the-scope colonic stents makes deployment proximal to the sigmoid technically challenging. Data are very limited in this context. A case series of 11 BDS for treatment of CD strictures of the terminal ileum or colon (deployed through overtube, assisted by a stiff guidewire, and fluoroscopy guidance) revealed high technical success (90.9%), but early stent migration occurred in 3 patients [134].
Henceforth, limited available data do not support endorsement of SEMS placement in the context of benign colonic conditions and should only be considered in case-by-case basis after multidisciplinary discussion. Recom-mendation: SEMS placement in benign colonic strictures should not be routinely performed.
Conclusion
Endoscopic stenting practices and techniques are continuously evolving, requiring clinicians to be aware of updated evidence in this field (Table 2). For patients with unresectable esophageal cancer, SEMS placement is recommended as a palliative measure if expected survival is less than or equal to 3 months. If available, brachytherapy should be considered as an adjunct for patients with expected survival above 3 months. SEMS placement is also recommended for patients with malignant tracheoesophageal fistulas as well as patients with RBES and transmural defects. Gastroduodenal stenting should be considered in patients with malignant GOO, especially those who have a short life expectancy (below 6 months). Colonic SEMS is the preferred treatment for palliation of malignant colonic obstruction and can be considered as bridge to surgery in selected patients. In all cases, individualized considerations and the multidisciplinary context should be made when developing management recommendations and plans.