Endoscopic access to the native papilla in patients with Roux-en-Y gastric bypass (RYGB) can be challenging when managing pancreaticobiliary disease. The endoscopic ultrasound (EUS)-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE), a technique first described in 2013 by Kedia et al. [1], consists in the creation of a temporary fistula between the gastric pouch, or the proximal jejunum, and the excluded stomach, by placing a lumen-apposing metal stent (LAMS) under EUS guidance.
We present a case of a 59-year-old woman, with a previous personal history of RYGB (grade III obesity). She developed an acute calculous cholecystitis and was submitted to laparoscopic cholecystectomy. A magnetic resonance cholangiopancreatography, performed 1-month after surgery, showed residual choledocholithiasis. After a multidisciplinary discussion, EDGE was proposed.
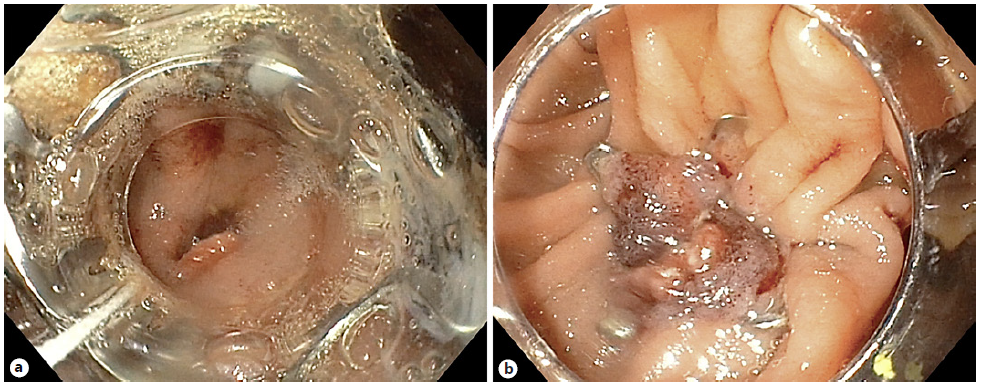
The procedure was performed in two stages, both under deep sedation, orotracheal intubation, and fluoroscopic control (video). In the first phase, a linear echoendoscope (GF-UCT180; Olympus Medical Systems, Japan) was advanced to the gastric pouch to localize the excluded stomach. When the closest point between the gastric pouch and the excluded stomach was located, a transmural puncture was performed, using a 19-G aspiration needle (ExpectTM; Boston Scientific®, Marlborough, MA, USA). Then the needle stylet was withdrawn, and 300 mL water-soluble contrast medium was injected into the excluded stomach, using EUS and fluoroscopic control. A 10 × 15-mm LAMS (HotAxiosTM; Boston Scientific®) was used to create an access to the excluded stomach: the distal flange was deployed under fluoroscopic and EUS guidance, and the proximal flange was deployed under endoscopic visualization into the remnant gastric pouch (Fig. 1).
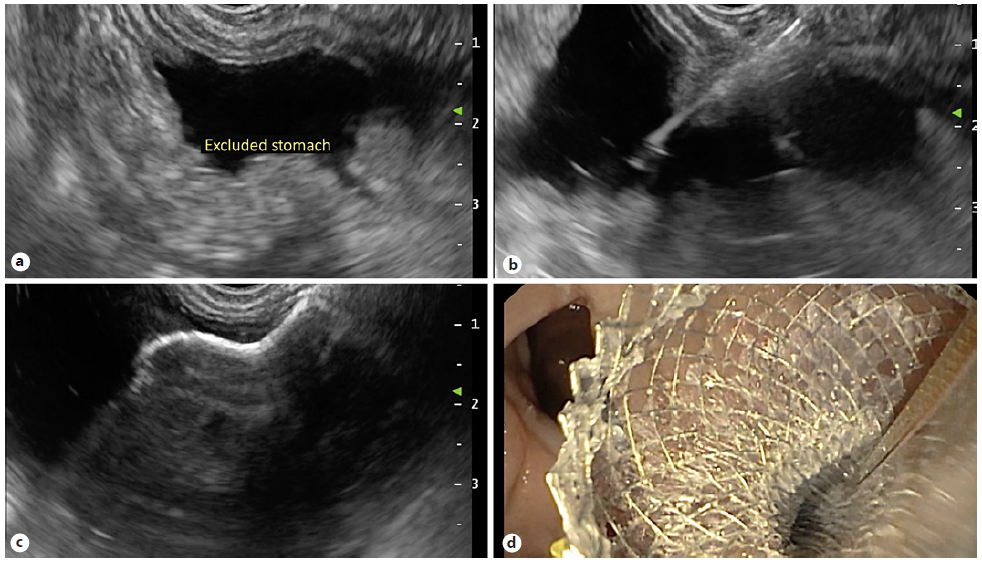
Fig. 1 First phase of procedure. a Echoendoscopic localization of the excluded stomach. b Transmural puncture with water-soluble contrast injection into the excluded stomach. c, d Deployment of the LAMS to create a gastro-gastric fistula.
Three weeks later, after gastro-gastric fistula maturation, the second stage was performed: a standard duodeno-scope (TJF-Q190V; Olympus Medical Systems, Japan) was advanced through the LAMS into the excluded stomach and then passed in an antegrade manner to the major papilla (Fig. 2). Due to a mature fistula and a fully expanded stent, there was an easy duodenoscope passage through the LAMS, obviating the need for balloon dilatation. A conventional ERCP was performed, with bile duct stone removal. After the ERCP was completed, the duodeno-scope was removed carefully, to prevent stent migration. The patient showed no immediate or late complications. Four weeks later, the LAMS was removed, and the fistula was closed with an over-the-scope clip (OTSC®, Ovesco Endoscopy, California, USA) (Fig. 3).
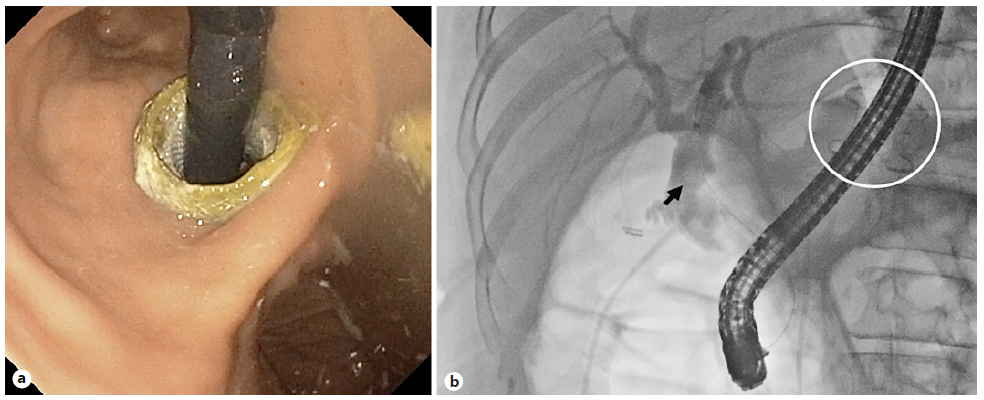
Fig. 2 Second phase of procedure, 3 weeks later: ERCP was performed through the LAMS (white circle), with bile duct stone removal (black arrow). a Endoscopic view. b Fluoroscopic control.
EDGE is a new technique with high technical (95.5-100%) and clinical success (95.9-98%) rates, compared to the ones of laparoscopy-assisted ERCP (LA-ERCP) (95.3% and 92.9%, respectively) and superior to enteroscopy-assisted ERCP (71.4% and 58.7%, respectively) [2-4]. EDGEs’ adverse events (AE) occur in 15.7-27.8% of cases. Most AE are minor, related to stent migration or misdeployment [2, 4, 5]. They are comparable to AE of laparoscopy-assisted ERCP (17.4%), but higher than those of enteroscopy-assisted ERCP (8.4%) [3, 4]. EDGE is a minimally invasive procedure that can be performed in a single session, if necessary. The 20-mm LAMS may be associated with reduced stent migration rates and higher single-session ERCP, but further studies are needed [2, 5]. We describe the EDGE technique step-by-step, a procedure with high clinical and technical success rates and with an acceptable safety profile, as it is an option when ERCP is mandatory in patients with RYGB.