Introduction
In December 2019, Wuhan city of Hubei province, China, reported a cluster outbreak of viral pneumonia that was subsequently confirmed to be caused by a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by it was termed coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [1, 2].
COVID-19 was considered a pandemic by WHO in March 2020. In Portugal, the first documented cases were confirmed on March 3, and after that, the number of infected people increased steadily, leading to the declaration by local authorities of a state of emergency since March 18, 2020.
The most common symptoms of COVID-19 are fever, fatigue, and respiratory tract symptoms such as cough and shortness of breath. Gastrointestinal symptoms, mainly diarrhea and vomiting, have also been reported [2-5].
The current available evidence suggests that SARS-CoV-2 is primarily transmitted through respiratory drop-lets and contact routes [1, 3, 5-9]. Airborne transmission may also be possible during procedures that generate aerosols such as gastrointestinal or respiratory tract endoscopies [1, 10, 11].
The transmission of SARS-CoV-2 in asymptomatic patients is one of the key factors responsible for its rapid dissemination across the world [1, 12]. There are several routes of transmission of COVID-19 in endoscopy units, which include person-to-person via direct contact (as endoscopy involves close contact with the patients or respiratory droplets), generation of infected aerosols during endoscopy, and contact with contaminated endoscopic equipment, accessories, and body fluids [13]. Theoretically, a patient with high-viral load in the respiratory secretions can contaminate the air of the endoscopy room. Fomites loaded with virus can remain viable for a longer duration, thus putting uninfected patients as well as endoscopy staff at risk [10].
Early on during the course of the pandemic, in order to limit the spread of COVID-19 and to protect both patients and healthcare workers (HCWs), multiple scientific societies around the world recommended that only urgent and high-priority endoscopic procedures should be done. In contrast to the severe acute respiratory syndrome outbreak in 2003 which was contained within 8 months [14], the COVID-19 pandemic has been exhibiting a vastly different epidemic trajectory. Maintaining a suitable balance between protecting HCWs and patients on the one hand and providing a timely and effective clinical service on the other hand have become more and more important as this pandemic persists [15].
Different pre-endoscopy screening strategies have been adopted around the world since the beginning of pandemic. Currently, as a screening strategy, questionnaires (symptoms and risky contacts) and RT-PCR testing for SARS-CoV-2 are used throughout several different endoscopy units globally prior to endoscopies.
According to the National Health Institute [16], all patients proposed for endoscopic exams, as an outpatient, must be screened and classified, using telephone questionnaires or equivalent, regarding the risk of COVID-19 in two moments: on the eve of the exam, by telephone, and on the day of the exam, before admission to the endoscopic unit. Despite not being recommended by the National Health Institute, the National Anesthesiology Society [17] recommends performing RT-PCR testing for SARS-CoV-2 in all patients before surgical or similar procedures in which it may be necessary to manage the airway (procedures under general anesthesia or anesthetic sedation).
And so, altogether, emerging evidence suggests that gastrointestinal endoscopy appears to be safe both for patients and HCWs if strict infection prevention and control measures are taken. Hence, our study aimed to assess the safety of endoscopy units during the COVID-19 pandemic, as well as the effectiveness and the need for SARS-CoV-2 screening prior to endoscopic exams.
Material and Methods
Study Design and Patients
We performed a retrospective, observational, and single-center study at the gastroenterology department of a Portuguese tertiary hospital. We included individuals who underwent elective endoscopic exams between September 1, 2020 and February 28, 2021. Patients with confirmed SARS-CoV-2 infection within 20 days prior to endoscopy were excluded. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local Ethics Committee.
Data Collection
Medical records and the Trace COVID-19 platform were reviewed. Trace COVID-19 is a Portuguese software, developed dur-ing the pandemic, which registers data from patients infected with SARS-CoV-2, including the date of diagnosis and all the other clinical and demographic data.
The following clinical and demographics parameters were con-sidered for analysis: age, gender, previous SARS-CoV-2 infection, endoscopic procedure, SARS-CoV-2 screening, infection by SARS-CoV-2 within 14 days after endoscopic exam. In order to investigate post-endoscopy SARS-CoV-2 infection, all patients were checked on the Trace COVID-19 platform, and the presence of a diagnosis of SARS-CoV-2 infection was verified within 14 days of the endoscopy procedure. All the parameters were analyzed and described for purposes of population’s characterization.
SARS-CoV-2 Screening Strategy
The SARS-CoV-2 screening performed was based on a specific questionnaire of symptoms related to COVID-19 and assessment of risk contacts with people infected with COVID-19 during the days prior to endoscopy or RT-PCR test for SARS-CoV-2 (nasal and oropharyngeal swab). The questionnaire was carried out up to 3 days before the endoscopy, and the RT-PCR test for SARS-CoV-2 was done within the previous 72 h.
All the patients who underwent endoscopic procedure under anesthetic sedation did only a RT-PCR test for SARS-CoV-2, and all other patients were asked the specific questionnaire. All patients who answered “yes” to any of the questionnaire topics did not undergo the scheduled endoscopic examinations, which were, then, postponed for at least 14 days.
On the day of endoscopy, all patients, before entering in the endoscopy unit, answered a brief symptom questionnaire, and the tympanic temperature was measured. In addition to the tests and questionnaires, all patients and professionals always wore adequate masks, regular hand disinfection was carried out, and body temperature was determined on entry into the endoscopy unit. The access of family members and caregivers to the endoscopy unit was also limited in accordance with local and national health authority recommendations. It is also worth noting that during the study period, it was no longer recommended to postpone non-urgent elective activity, and therefore, all exams were being re-sumed.
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences® (SPSS), version 24.0. For categorical variables, the authors present frequencies (n) and percentages (%). For continuous variables with symmetric distribution, we determined means and standard deviations. Intervals between prior SARS-CoV-2 infection and the endoscopic procedure and between endoscopy and the SARS-CoV-2 infection, the median, and interquartile range (IQ) were used. The assumption of normality was verified by the Kolmogorov-Smirnov test, through the values of asymmetry and kurtosis, as well as by the analysis of histogram graphs.
Results
Over the study period, a total of 2,166 patients underwent endoscopic exams. Patients had a mean age of 61.8 ± 14.7 years and were mostly male (56.2%, n = 1,218). None of the patients were vaccinated.
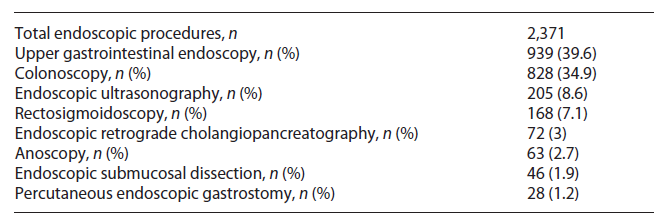
The exams were mostly performed in an outpatient setting, with only 118 (5.4%) patients requiring hospitalization after the endoscopic exam, namely, those undergoing endoscopic retrograde cholangiopancreatography and endoscopic submucosal dissection. Upper gastrointestinal endoscopy was the most common procedure (43.4%, n = 940). A description of the endoscopic procedures performed can be found in Table 1.
The main reasons for referral were extraction of colonic polyps (17.4%, n = 377), assessment of gastric dysplasia (7.5%, n = 162), diagnosis/follow-up of patients with inflammatory bowel disease (7.2%, n = 155), surveillance after colorectal cancer resection (5%, n = 109), and study of anemia (4%, n = 87). Gastric dysplasia represented an important part of the indication for performing endoscopic exams since it was considered a priority indication for upper gastrointestinal endoscopy during the COVID-19 pandemic and, also, because the study center is considered a reference center for endoscopic submucosal dissection, leading to an increase in the number of patients with this premalignant condition.
Eighty-one (3.7%) patients had previous SARS-CoV-2 infection, with a median difference of 74 days (IQ 40.5:160.5) between infection and endoscopy. Most patients (70.2%, n = 1,521) did an RT-PCR screening for SARS-CoV-2 up to 72 h before the procedure, with the remaining patients (29.8%, n = 645) answering a questionnaire of symptoms and positive risk contacts the day before the procedure; all of them presented a “negative” questionnaire.
Of the patients who underwent RT-PCR screening for SARS-CoV-2, 21 (1.4%) tested positive, and all were asymptomatic at the time of the screening. Endoscopic procedures for these patients were postponed at least 14 days.
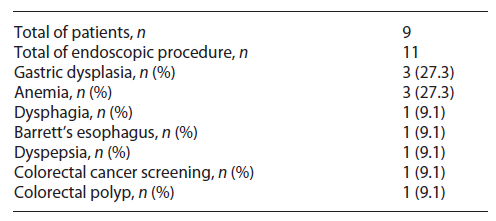
Nine patients (0.42%) with an initial negative RT-PCR screening test developed SARS-CoV-2 infection within 14 days after visiting our department. The median difference between the procedure and diagnosis of infection was 10 days (IQ 6.5-13). All these patients were confirmed to have a negative RT-PCR test before endoscopy. Their endoscopic procedures were performed in an outpatient setting: upper gastrointestinal endoscopy in 5 patients, colonoscopy in 2 patients, and upper gastrointestinal endoscopy plus colonoscopy in the remaining 2 patients. Table 2 summarizes clinical indication for endoscopies in these patients.
None of the patients submitted to the questionnaire had a SARS-CoV2 infection within 14 days after the endoscopic exam. It should also be noted that during the study period, none of the HCWs of the endoscopy unit became infected with SARS-CoV-2.
Discussion
The pre-endoscopy SARS-CoV-2 screening strategy should carefully analyzed. The two main concerns with this pretesting strategy are the false-positive and the false-negative tests. An infected individual with a negative RT-PCR test (false negative) using a surgical mask entering an endoscopy unit may be hazardous for staff and other patients. On the other hand, a patient with a false-positive test will have their exams called off and enter a 10-day quarantine period with absenteeism and consequent increase in anxiety and apprehension [18].
In our study, the prevalence of SARS-CoV-2 infection in patients undergoing pre-endoscopy screening was 1%, all of them asymptomatic and detected by RT-PCR test for SARS-CoV-2, which is in agreement with the few recent available studies [19-22]. These studies showed that asymptomatic SARS-CoV-2 among patients referred for endoscopic procedures had a prevalence ranging from 0.0% to 1.5%, but most studies reported a range from 0% to 0.5% regardless of local surges of COVID-19 cases. In all of these studies, the importance of symptom screening in endoscopy units is emphasized.
Regarding the prevalence of SARS-CoV-2 infection after endoscopic exams, we identified a prevalence of 0.42% of SARS-CoV-2 infection within 14 days after endoscopic exam. Once again, these findings are consistent with published literature. Several studies [23-30] show that post-endoscopy rates of infection ranged from 0% to 0.4%. The cases of COVID-19 were attributed to endoscopy exposure if there was no other reasonable justification. However, this assumption may be a bias, overestimating infection and transmission. Of these studies, 5 were in the context of a pre-procedure testing strategy, and 3 did not have an explicit pre-procedure testing strategy.
Several studies have already been carried out and demonstrated the effectiveness of vaccination in reducing the transmission of SARS-CoV-2. In a study carried out in Israel [31], the viral load present in the nasal mucosa of a sample of HCWs was evaluated on a weekly basis. The conclusion of the study showed that in vaccinated persons with COVID-19 infection, the viral load was 2-4 times lower than in unvaccinated persons. Also, another study performed in the USA [32] conducted on a sample of 3,950 HCWs showed that vaccines had an efficacy in preventing infection of 90% in the 14 days after the second dose and 80% in the 14 days after the first dose.
Recent guidelines published by the American Gastroenterological Association, concerning SARS-CoV-2 testing and endoscopy postvaccination [33], acknowledged the small potential benefit of pre-procedure testing (using RT-PCR test for SARS-CoV-2) with respect to patient and staff reassurance. However, there was no apparent benefit from preventing infections. The panel also evaluated the yield of testing and significant delays in care as well as decreased number of diagnoses of gastrointestinal cancers. Therefore, more value should be given to avoid delays in care, leading to a downstream impact on cancer diagnoses and other important diseases. Multiple visits to clinical units are also a factor also to be taken into consideration, and yet, avoiding all these unnecessary tests could lead to a great saving of time, money, and resources, with a major impact on our healthcare system.
Some limitations need to be mentioned: a causal relationship between an endoscopic procedures and subsequent SARS-CoV-2 infection could not be fully proved, and therefore, our results may be overrated. Furthermore, our investigation was single-centered, and data collected retrospectively; and therefore, some data may have been lost. These data may include patients with a positive symptom screening which, consequently, were no longer included in the list of endoscopies to which the authors had access. For this reason, patients who underwent symptom screening were not included in the rate of positive screening since this probably would lead to an underestimate value.
In Portugal, different hospitals followed different strategies with many clinical facilities performing only symptom screening. Hence, large prospective and multicenter trials could play an important role to validate our findings and defining the best pre-endoscopic screening approach in the future, for this or another pandemic scenario.
This was a pioneering study on the safety of endoscopic units during the COVID-19 pandemic in Portugal. There are no other Portuguese records assessing the inci-dence of SARS-CoV-2 infection after endoscopic procedures.
In conclusion, pre-endoscopy screening with RT-PCR test for SARS-CoV-2 identified a very small number of patients with SARS-CoV-2 infection, all of them asymptomatic and therefore with low risk of transmission [34]. Moreover, the number of patients with SARS-Cov-2 infection in the 14 days following endoscopy was very low, and this number may be even lower since it is not possible to fully associate these infections with the hospital visit. Given the current high vaccination rate (90%), we assume that screening of symptoms and identifying risk contacts is important and may be sufficient to prevent infection with SARS-CoV-2, if protective equipment is adequately used. That said, our study allows us to conclude that endoscopy units were safe, both for patients and HCWs during the COVID-19 pandemic and that, according to the most recent literature, pre-endoscopy SARS-CoV-2 screening should be rethought and standardization of actions should be applied across the country.