Introduction
Endoscopic ultrasound (EUS)-guided sampling is regarded as a safe and accurate diagnostic tool for the evaluation of mediastinal lymphadenopathies and masses of unknown origin [1]. The overall incidence of adverse events associated to EUS-guided puncture is very low, and the most common adverse event is infection (pooled infection rate, 0.4-1.7%), which is significantly higher for cystic lesions compared to solid ones [2, 3]. Limited evidence is available regarding patient-related risk factors of adverse events associated to EUS-guided sampling [2]. Awareness of such risk factors is crucial in the decision-making process of sampling (when and how to puncture), in the definition of preventive strategies, and in the optimization of the informed consent process. The only well-established risk factor for infection associated to EUS-guided sampling is the puncture of cystic lesions (specifically, pancreatic cysts or mediastinal cysts) [2]. Data from a large retrospective series, including 252 patients with sarcoidosis, documented an increased risk for mediastinal abscess formation and mediastinitis after EUS-guided sampling in patients with sarcoidosis (30-fold higher than for other indications for EUS-guided nodal sampling in the mediastinum) [4]. The authors describe a case of a young patient with a final diagnosis of sarcoidosis, who developed a large mediastinal abscess after EUS-guided fine-needle biopsy (FNB) and discuss potential preven-tive measures to avoid such severe complication.
Case Report
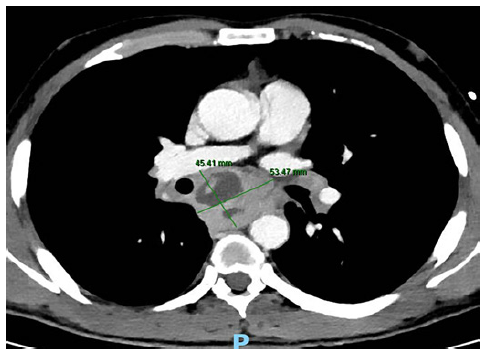
A 38-year-old man with a past history of a surgically removed duo-denal GIST (AJCC stage 1, R0) was referred for EUS-guided sampling for characterization of several enlarged mediastinal lymph nodes, documented on computed tomography (CT). The patient was asymptomatic and had no lung lesions or abdominal lymphadenopathies on CT. EUS documented several coalescent, crescent-shaped lymph nodes in the posterior mediastinum, with a hypoechogenic homogeneous pat-tern, the largest with 35 × 17 mm in the subcarinal station (Fig. 1). FNB was performed using a 22-gauge fork-tip needle (SharkCore; Medtronic, Sunnyvale, CA), with three dedicated passes (until obtaining a macroscopic visible whitish core), using the fanning and stylet retraction techniques. Polymerase chain reaction for Mycobacterium tuberculosis and assessment of clonal B cell populations by flow cytometry were negative, and noncaseating granulomas with multinucleated giant cells, compatible with sarcoidosis, were documented on pathology (Fig. 2). Two weeks after EUS-FNB, the patient was admitted due to increasing retrosternal pain, fever (39°C), and progressive dysphagia. Serum inflammatory markers were elevated, and chest CT revealed a large subcarinal mass (54 × 45 mm) with heterogeneous liquefactive areas, consistent with a mediastinal abscess, in continuity with a thickened esophageal wall (Fig. 3). The patient was treated with intravenous meropenem for 7 days, followed by prolonged (4 week) oral treatment with ciprofloxacin (750 mg b.i.d.) and metronidazole (500 mg t.i.d.), with progressive clinical improvement and recovering completely. Treatment duration was based on previously reported cases, where noninvasively treated patients required prolonged (at least 4-week course) antibiotic therapy [4, 5].
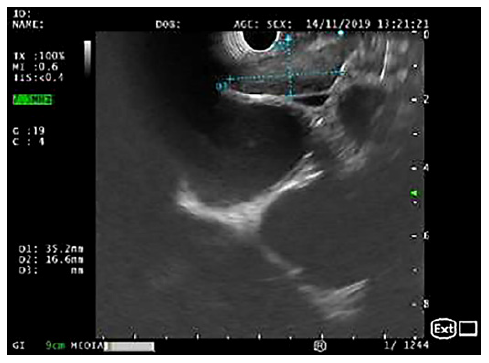
Fig. 1. EUS (linear array, transesophageal view - station 7): crescent-shaped lymph node, with 35 × 17 mm and a hypoechogenic homogeneous echo pattern.
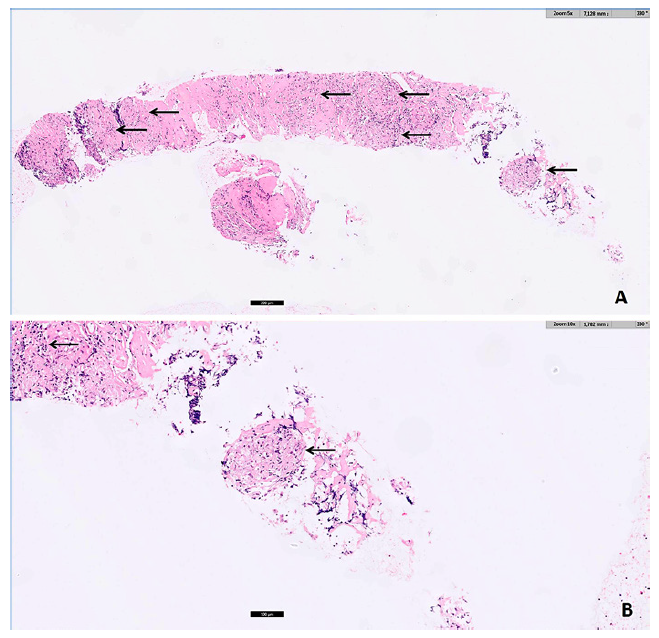
Fig. 2. Pathology (A H&E, ×5; B H&E, ×10): noncaseating epithelioid granulomas (arrows) with multinucleated giant cells, in a fibrotic stroma, compatible with sarcoidosis.
Discussion
Current guidelines point out a higher risk of infection after EUS-guided sampling of mediastinal cystic lesions rather than of solid lesions [1-3]. As mediastinal abscess and mediastinitis are associated to high morbidity and potential mortality, EUS-guided sampling of mediastinal cysts is globally discouraged and should be restricted to carefully selected cases [1-3]. In patients undergoing EUS-guided sampling of any cystic lesion, prophylactic antibiotic administration is recommended [1-3]. Although infection prophylaxis is not advocated for EUS-guided sampling of solid lesions (as the pooled infection rate is very low) [1-3], a retrospective case series [4] and a systematic review [5] documented an increased risk of infection, with mediastinal abscess formation, after EUS-guided puncture of lymph nodes in patients with sarcoidosis.
Sarcoidosis is a granulomatous disease affecting mostly young adults and presenting with mediastinal or hilar lymphadenopathies in 85% of cases [4]. In suspected sarcoidosis, EUS-guided sampling of mediastinal nodes is increasingly being used as it demonstrated to have higher diagnostic yield when compared to conventional bronchoscopy with transbronchial and endobronchial biopsies in the so-called Granuloma trial [6] and a similar diagnostic yield when compared to endobronchial ultrasound-guided sampling in the multi-center International Sarcoidosis Assessment (ISA) trial [7] (even though only first- and second-generation FNB needles were used in the EUS arm [7]). In a large series of 252 patients with the final diagnosis of sarcoidosis undergoing EUS-guided sampling, 5 patients developed mediastinal abscesses and 4 of those patients required surgical drainage [4]. This corresponded to a 30-fold higher incidence of nodal infection after EUS-guided puncture in patients with sarcoidosis compared to patients submitted to mediastinal node sampling for other indications (mostly for lung cancer staging) in the same institution [4]. An increased risk for infection after lymph node puncture in patients with sarcoidosis (caused by iatrogenic inoculation of commensal flora by the needle) may be related to the distribution of regulatory T-cells at the periphery of sarcoid granulomas, which may account for the state of anergy (poor response to antigens in vitro and in vivo) that characterizes sarcoidosis [8]. Endoscopists should be aware of the potentially increased risk of infection when sampling mediastinal lymph nodes in suspected sarcoidosis and a strategy to minimize such risk should be followed, although current guidelines do not yet consider this issue. An easy strategy to minimize the infection risk may be reducing the number of needle passes, by selecting a third-generation (frontal cutting) FNB needle (with a higher diagnostic yield per pass), and using onsite evaluation to confirm sample adequacy and the presence of granulomas, thus dismissing additional passes [9-11]. The use of prophylactic antibiotics for EUS-guided mediastinal lymph node puncture in suspected sarcoidosis should also be considered, and this approach is presently followed by the authors, using an antibiotic regimen similar to the one recommended for cystic lesions puncture [2, 3]. The low frequency of infection after lymph node sampling in the suspicion of sarcoidosis (∼2% [4]) would require large numbers in
trials to achieve adequate statistical power, and prospective studies validating the potential benefit of antibiotic prophylaxis in this setting are unlikely to be undertaken. Since endobronchial ultrasound-guided sampling seems to have a negligible risk of infection in sarcoidosis, which may be related to a lower contamination rate of commensal flora by the needle through the respiratory tract, this sampling route may be considered in this setting [5, 7].