Introduction
Acute liver failure (ALF) is defined by acute liver injury with hepatic encephalopathy (HE) and an elevated international normalized ratio (INR above 1.5). Normally, HE presents on the first 8-28 days of jaundice. Drug-induced and viruses, with high prevalence of hepatitis B virus (HBV), are currently the main causes [1]. Severe HBV-related ALF has a spontaneous survival rate of 25% [2]. Pathophysiology includes the accumulation of various metabolites and toxins, namely, ammonia, that lead to cerebral edema and herniation. Additionally, a systemic inflammatory response (SIRS) occurs and is responsi ble for multiorgan failure (MOF), namely, acute kidney injury (AKI), which happens in 40-80% of the cases [1, 3, 4].
ALF should be managed in an intensive care unit (ICU) at a liver transplant center. The approach involves treating the cause of the hepatitis as well as organ support measures. Early consideration should be given to the indication for liver transplantation (LT) as well as the artificial liver support system (ALSS). The presence of hyperammonemia and HE in ALF could be an indication for continuous renal replacement therapy (CRRT) [1]. CRRT, continuous venovenous hemodiafiltration (cv-vHDF) included, allows the clearance of ammonia and improves 21-day transplant-free survival, as found by Cardoso FS et al. (2018) [5]. In the ALSS group, high-volume plasma exchange (HVPE) has demonstrated benefit in increasing transplant-free survival at 3 months, as found by Larsen FS et al. (2016) [6]. HVPE is considered a rescue therapy when initial measures fail, allowing ammonia and inflammatory mediator clearance, which are responsible for MOF and, consequently, mortality [7].
Therefore, both are promising therapeutic weapons for patients who are not candidates for LT, allowing an opportunity for liver recovery and mitigating the need for LT. We share a case of fulminant acute hepatitis B virus with LT contraindication in which the institution of s-quential HVPE and CRRT allowed noticeable clinical and hepatic improvement.
Clinical Case
A 51-year-old male was admitted in the emergency department for jaundice with 7 days of evolution. Personal history of sporadic consumption of alcohol was identified. At physical examination, mucocutaneous jaundice was found. Blood samples revealed elevation of liver cytocholestasis markers (aspartate transaminase 6,688 U/L; alanine transaminase 6,223 U/L; gamma-glutamyltransferase 243 U/L; and alkaline phosphatase 188 U/L), liver dysfunction (INR 2.6; total hyperbilirubinemia [TB] 27.12 mg/dL; and direct bilirubin 19.53 mg/dL; factor V 46.6%; ammonia 219 μg/dL), and mixed alkalemia (pH 7,47; PaCO2 32.5 mm Hg; HCO3 23.7 mEq/L; lactate 1.7 mmol/L). An abdominal-pelvic computed tomography scan showed an expansive lesion in the left kidney, grade IV of the Bosniak classification. No changes related to the liver, bile ducts, spleen, or pancreas were described. In the etiological study performed, positive AgHBs, AcHBc IgM, and AcHBe with low viral load (VL) HBV of 41,100 IU/mL were found. Serology for hepatitis D virus was negative.
A diagnosis of severe acute hepatitis B and probable solid neoplasm were assumed. The patient was transferred to the ICU and started on N-acetylcysteine and entecavir at a dose of 1 mg/day. After 48 h in the ICU, ALF criteria were met. Progression for grade IV HE motivated orotracheal intubation. Preventive measures of cerebral edema were instituted, and monitorization of intracranial hypertension was initiated with the measurement of the optic nerve sheath. Concomitantly, liver tests worsened, with a maximum TB of 60.59 mg/dL and direct bilirubin of 37.78 mg/dL; INR of 7.9; hyperammonemia of 423 μg/dL; and hyperlactacidemia of 2.3 mmol/L. No other organ failure was identified.
Although the Kings College and Clichy criteria for LT had been met, the probable malignant neoplasm represented a contraindication. Due to the persistent neurological dysfunction and worsening of liver tests, the team decided to start HVPE. A filter membrane was chosen and 8 L of plasma was infused in each of the 3 sessions. The median duration of each session was 8 h. The replacement volume was equal to the treated volume. To reduce the risk of bleeding and infection, the replacement volume consisted of 5% human albumin and fresh frozen plasma (FFP) in a ratio of 1:1. During HVPE, AKI was identified, with creatine 1.51 mg/dL, and terlipressin was started.
At the end of the third HVPE session, worsened AKI (maximum creatinine 3.26 mg/dL and urea 74 mg/dL) and hyperammonemia (325 μg/dL) led to the initiation of cvvHDF. An effluent dose (ED) of 50 mL/kg/h was pro-grammed, without losses. There were no identified any classic indications for CRRT. After 4 days of cvvHDF, the ammonia level was under 100 μg/dL, and CRRT was suspended.
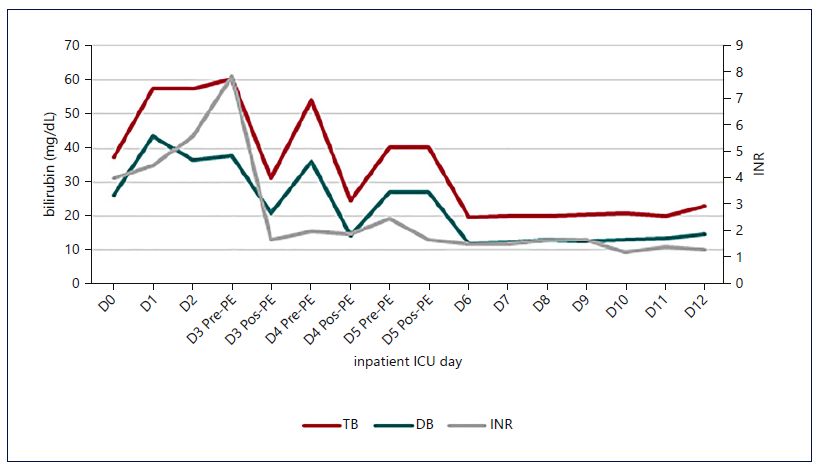
Fig. 1. Evolution of INR and bilirubin with the institution of HVPE and cvvHDF. A decrease in liver function markers was notice, which was maintained after discontinuation of the techniques. The stabilization of the INR value occurred earlier than that of bilirubin.
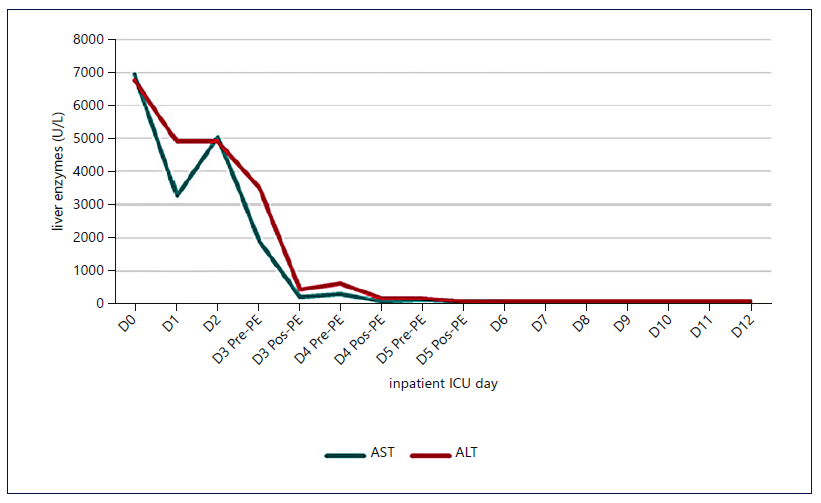
Fig. 2. Evolution of transaminases with the institution of HVPE and cvvHDF. A significant and early drop in transaminases was identified, which was maintained.
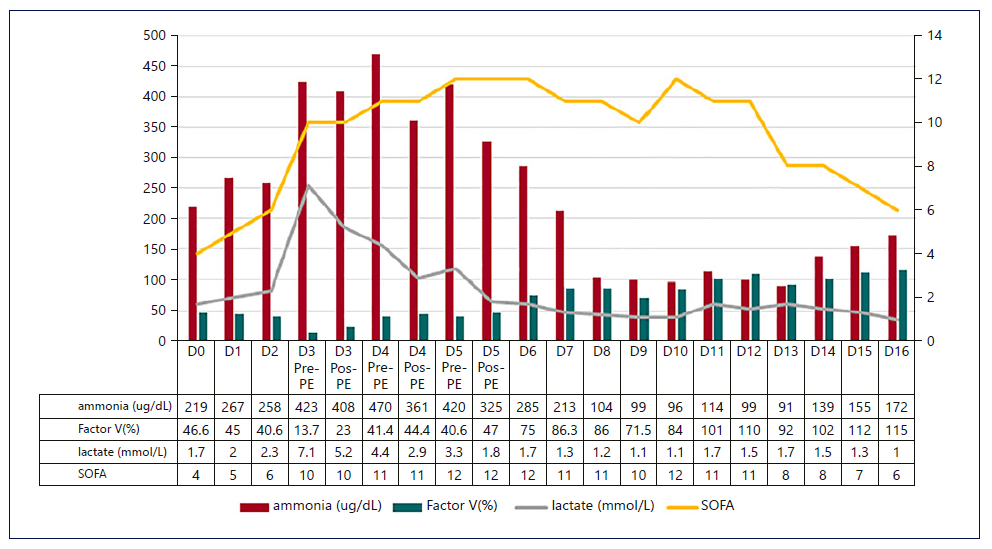
Fig. 3. Evolution of SOFA score, serum lactate, ammonia, and FV with the established techniques. The SOFA score and ammonia followed the evolution of the previously described markers, until the infectious intercurrence. PE, plasma exchange; SOFA, Sequential Organ Failure Assessment; FV, factor V.
Figures 1-3 show the evolution of liver tests, ammonia, lactate, and sequential organ failure assessment (SOFA) score. After three HVPE sessions and 4 days of cvvHDF, ammonia levels and liver tests achieved sustained improvement. FFP administration is a confounding factor for the factor V elevation.
The mortality predicted by the SOFA score achieved the maximum value of 95.2% on the 5th day in the ICU, as shown in Figure 3. After discontinuing cvvHDF, the predicted mortality decreased, following the evolution of liver tests and ammonia, as shown in Figures 1-3.
On the 10th day in the ICU, a ventilator-associated pneumonia was diagnosed, and a second maximum value of predicted mortality was achieved. Concomitantly, hy perammonemia occurred, but ammonia level normaliza-tion was observed without invasive measures.
After 3 days without cvvHDF, the patient was extubated. Clinical and analytical improvement was noticed. The patient was submitted to robot-assisted left radical nephrectomy on the 22nd day in the ICU. The histology of specimen revealed a clear cell renal carcinoma, grade 2 of grading system ISUP/OMS.
The patient was transferred to an internal medicine ward for continuity of care on the 31st day in the ICU. At that time, he had a Glasgow coma scale score of 15 points without sequels beyond ICU-associated myopathy. Analytically, he had cytocholestasis markers within reference values; TB 5.75 mg/dL; INR 1.4; lactate 0.7 mmol/L; creatinine 1.66 mg/dL; urea 30 mg/dL; and a HBV VL 51 IU/mL. Four months after hospital admission and a complete program of physical rehabilitation, the patient has fully recovered his autonomy.
Discussion
This case illustrates a new approach to HBV-related ALF with sequential use of HVPE and cvvHDF, with a significant improvement in liver tests without LT. The absence of immunosuppressive therapy or recent risk fac-tors for HBV transmission suggests that ALF was caused by spontaneous HBV reactivation. Most reactivations are related with immunosuppression. Since kidney cell cancer is associated with immunosuppressive phenotype by upregulation of transforming growth factor β, its possible contribution was considered. However, scientific evidence regarding this association is scarce [1, 7-10].
Among the therapeutic measures instituted, antiviral therapy should be highlighted. The purpose of entecavir in acute hepatitis B is to prevent progression for ALF, but its role when ALF is already established is questionable [11]. Entecavir was the drug of choice since it is highly effective in reducing HBV VL [2, 11-13]. About 13% of the circulating entecavir molecule is bound to albumin; however, information on the effect of HVPE and CRRT in entecavir bioavailability is limited [13]. Even with HVPE and cvvHDF sessions, suppression of HBV viral replication was verified, with a reduction in 99% of HBV VL, on the 20th day of entecavir.
ALF criteria were met in the ICU, and despite having an indication for LT, the existence of an untreated extra-hepatic cancer represented a formal contraindication [9]. Non-indication for LT did not imply nonadmission in the ICU. Therapeutic and support measures instituted, namely, neurological and respiratory, are associated with improved survival. Various indicators of poor prognosis were identified in this case. HE reflects a severe alteration of hepatic function, with hyperammonemia being a reliable marker of HE and is also associated with intracranial hypertension and brain herniation if above 150-200 μg/dL [1, 14-16].
Additionally, AKI developed; serum lactate elevation and hyperbilirubinemia suggested an unfavorable outcome [1, 9, 14, 15]. That, in the presence of a persistent neurological dysfunction and a contraindication for LT, led the team to institute other invasive measures, such as HVPE and cvvHDF. The tandem cvvHDF-HVPE has been used in immune-mediated AKI, without hemodynamic or ionic complications described, although it has not been used in ALF. In that setting, the authors decided to use a sequential approach [17, 18].
Due to the persistent neurological dysfunction and worsening liver tests, it was decided to start HVPE in the first place. It was chosen as the first option because of its effect, allowing not only ammonia clearance as well as bilirubin and others inflammatory mediators, the latest related with MOF and mortality. Among therapeutic plasma exchange techniques, HVPE is the 1st option in ALF treatment [2-4, 19, 20]. Compared to other ALSS, namely, molecular adsorbent recirculating system or Prometheus, HVPE has an advantage in transplant-free survival, as demonstrated in the prospective multicenter study of Larsen et al. (2016) [1, 6, 7, 19-21]. HVPE consists of separation of plasma from other blood components, then its removal, followed by the administration of a replacement solution. The difference from other therapeutic plasma exchange is related to the volume of total plasma treated. In HVPE, 8-12 L of plasma volume are removed, which corresponds to 15% of the ideal body weight. The removal rate is 1-2 L per hour, instead of 1-1.5x of the total plasma volume [1, 4, 9, 19, 22].
The replacement solution may consist of albumin and/or FFP [22]. The difference between the biochemical effect of albumin and FFP is still not fully understood, nev-ertheless the risk of infection and bleeding associated with each of the solutions are well defined. FFP allows the replacement of all plasma components. Nevertheless, FFP is expensive, requires blood type compatibility, and has the risk of viral infection and citrate toxicity. On the contrary, albumin has no infectious risk but leads to a decline in other plasma components, such as coagulation factors, and more prolonged depletion of all antibodies, especially IgG antibodies. Immunoglobulins replacement after HVPE was not considered as it is expensive and in FFP composition, there are immunoglobulins and complement components [7, 23]. To equilibrate the risks of both solutions and reduce the costs, the authors decided to use a replacement solution composed of 50% albumin and 50% FFP. In Larsen et al. [6] study, patients with active malignancy were excluded, and the replacement volume used only consisted in FFP. The alternative replacement solution used in this case allowed achieving a good result. The HVPE duration was limited to 3 consecutive days due to the infectious risk, with intervals of 24 h, allowing regeneration of clotting factors and reducing the bleeding risk [5, 6, 19, 22].
Despite the improvement seen after the third HVPE session, worsening of AKI and hyperammonemia (3 times above the UNL), led the team to start CRRT, which is an ammonia-lowering strategy. Early CRRT use could be indicated in ALF in the presence of persistent hyper-ammonemia and hyponatraemia and for temperature control, even in the absence of classic indications for RRT [1, 5, 16, 24, 25]. The choice of cvvHDF over the intermittent technique is related with his minimal rebound effect, contributing to persistent improvement of ammonia levels [16, 26]. The authors chose an ED of 50 mL/kg/h because ammonia is a large molecule and needs higher ED to allow its clearance [16, 27]. cvvHDF was stopped when ammonia levels were under 100 μg/dL, although some authors recommend to stop when values are under 70 μg/dL [16]. Most patients with ALF and AKI achieved fully renal function recover upon discharge [1]. Kidney cancer can contribute to AKI; however, the mechanism is due to glomerulopathy, which is rare, being systematic inflammatory response-ALF the main contributor in this case [1, 9, 25, 28].
Besides the bleeding risk, hypocoagulation with citrate was used in HVPE and in cvvHDF. The goal was to reduce the risk of filter clotting which is associated to lower filter performance and clearance commitment. Hypocalcae-mia is an adverse effect of citrate, so monitorization with arterial blood gas analysis is required. Compared to citrate, heparins have an increased risk of bleeding and required close monitorization for dose adjustment [29, 30].
The sequential use of HVPE and CRRT represented a game changer. Despite the poor prognostic and higher predicted mortality, an impressive liver recovery without LT was achieved. In a period when demand exceeds organic supply, the combination of both techniques appears as a good option, especially in patients with contraindication to LT.















