Introduction
Endoscopic submucosal dissection (ESD) is a recently differentiated minimally invasive endoscopic technique used for the resection of superficial gastrointestinal lesions that cannot be completely addressed by conven-tional endoscopic mucosal resection (EMR) nor are invasive enough to undergo surgery. According to the updated European Society of Gastrointestinal Endoscopy (ESGE) guidelines, ESD is the treatment of choice for several gastrointestinal lesions, including large pre-carcinomatous adenomas and early (T1) cancers. Indications depend on precise characterization of the lesion and its aggressiveness, which is preferably achieved by high-definition white-light and virtual chromoendoscopy, allied to internationally validated classifications such as the Paris, NICE, and JNET ones. In general, no other imaging study is required before dissection [1].
Comparing to EMR or surgery, ESD entails numerous advantages and limitations. Compared with EMR, ESD has higher en bloc and complete resection rates and lower recurrence rates. However, it implies a higher perforation risk, technical difficulty, and procedural time. Compared with surgery, ESD is associated with lower procedural time, length of hospital stays and costs, adverse events, and procedure-related morbidity and mortality. However, a higher recurrence risk and lower disease-free survival are generally associated [1, 2]. Therefore, an accurate decision based on the lesion’s resectability, the patient’s performance status, and the instrumentalist’s expertise is crucial [3].
This study focuses on giant ESD resections (>10 cm) located in the rectum. Currently, rectal ESD should be considered for lesions endoscopically suspected of only limited submucosal invasion (demarcated depressed area with irregular surface pattern or large protruded or bulky component) or that cannot be completely removed by EMR [1].
Giant ESD resections are usually challenging and have been associated with longer procedural time and higher adverse event rates (bleeding and perforation). The en bloc resection and curative rate have been comparable to smaller lesions, with no posterior salvage surgeries, strictures, local recurrences, or metastasis [2]. Numerous studies have discriminated tumor size, submucosal fibrosis, invasive depth, and procedure time as risk factors for complications and incomplete resections [4].
Despite increasing literature on giant resections, data on their efficacy and safety are still lacking. Overall, we propose to analyze our outcomes for rectal resections larger than 10 cm, comparing them to other international experiences. Additionally, we propose to identify any possible risk factors significantly associated with different outcomes or complications.
Materials and Methods
This is a single-center uncontrolled study conducted by the Gastroenterology Department at Centro Hospitalar de Lisboa Ocidental (CHLO) in Lisbon, Portugal. Consecutive patients with rectal resections larger than 10 cm submitted to ESD from January 2016 to December 2021 were included. A total of 15 rectal ESDs were performed. All patients were informed about the risks and benefits of ESD, and provided written informed consent. Patient information, follow-up exams, and consultations were obtained from their electronic medical record or telephonic contact. Outcomes included en bloc and curative resection rates, complications, the need for future endoscopic management, chemoradiation therapy or surgery, disease recurrence, and disease-related mortality. Statistical analysis for patient and lesions’ characteristics, outcomes, and identification of possible risk factors significantly related to them was done through Microsoft Excel (version 2018) and SPSS (version 29). Comparison with other international studies was obtained through search and evaluation of already published scientific articles in PubMed.
ESD Procedure
All ESD procedures were performed by the same therapeutic endoscopist (PB) in a onetime session. Patients were under general sedation and endotracheal intubation by an anesthesiologist. In longer procedures, patients were covered with blankets and warmed up to avoid hypothermia. A transparent cap on the endoscope tip and CO2 insufflation were used. Lesions were previously analyzed by white light and narrow-band imaging to detect signs of invasiveness. Submucosal injection was done with a mixture of 4% gelatin solution (Gelafundin 4%, B. Braun Melsungen AG, Germany), indigo carmine dye, and adrenaline (1:250,000). Electrocautery marking, mucosal incision, and submucosal dissection were performed using the Flush knife (Fujinon-Toshiba ES System Co., Omiya, Japan) alone or in combination with IT knife nano (KD-612L, Olympus). The electrosurgical ERBE ICC 200 generator unit (ERBE Elektromedizin, Tubingen, Germany) was set at “Endocut” (effect 3, 60 W) for mucosal incision, “Forced Coag” (45-55 W) for submucosal dissection, and “Soft Coagulation” for hemostasis (45-55 W). The scar’s visible vessels were routinely electrocoagulated to prevent delayed bleeding. By routine, multiple tunneling and patient mobilization methods were used. No traction or pocket-creation methods were used. For piece recovery, a large snare was routinely used with anal lubrification and digitation help. After the procedure, all patients stayed hospitalized for clinical surveillance according to protocol. Follow-up bloodwork or exams were requested only if complications were suspected. For endoscopic resections with more than 75% circumferential resection, prophylactic topical corticotherapy (budesonide) was used. Endoscopic reports included macroscopic description of lesions (location, size, and Paris and JNET classifications) and procedural description (time, methods, en bloc resection, early adverse events).
Histopathology Assessment
All ESD resected specimens were pinned down with needles, measured, fixed in 4% neutral buffered formalin, and sent to the pathology department. Pathology reports included dimension, histology (Vienna classification), differentiation, involvement of resection margins (RX resection), budding, and lymphovascular invasion.
Definitions
En bloc resection was considered when removal of the entire lesion in a single piece was achieved. Complete R0 resection re-ferred to ≥1 mm tumor-free horizontal margin and negative vertical margins on histology. Resections were classified as curative, non-curative with high-risk, or non-curative with local-risk recurrence, according to the updated ESGE criteria. Adverse events assessed included intraoperative or delayed perforation, bleeding, and stenosis. Intraoperative perforation was defined as an injured muscle layer with peritoneal cavity or fat tissue visualization identified during the procedure. Delayed perforation was defined as perforation manifested clinically (such as fever, abdominal pain, clinical instability) with imaging studies showing fluid collections and/or free intraperitoneal air. Bleeding was considered major if it was not controlled by endoscopy (need for surgery or radiological intervention) or if there was a need for transfusion or clinical instability. Minor bleeding included the need for change in the procedural plan (for instance, application of hemoclips) or >5 min for endoscopic control. Delayed bleeding referred to hematochezia or hemoglobin fall of >2 g/L in the first 30 days after ESD. Stenosis was defined as a luminal narrow and inability to pass a pediatric colonoscope or clinical symptoms of sub-occlusion/occlusion.
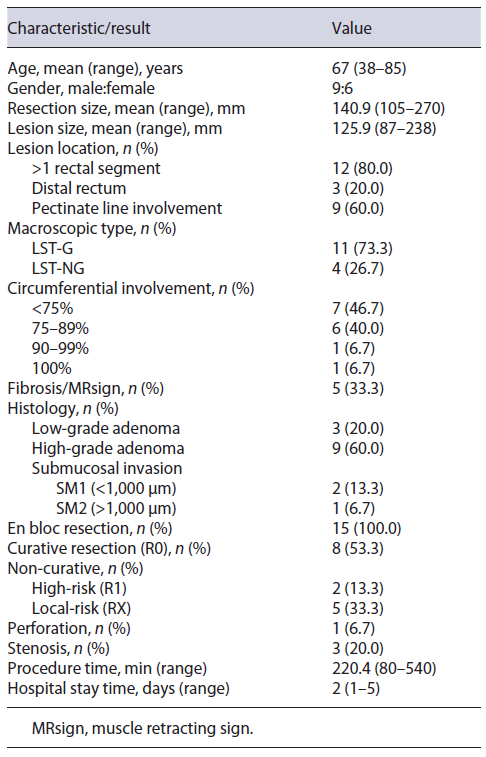
A total of 15 patients underwent giant rectal ESD resections between January 1st, 2016, and December 31st, 2021, at our center. The clinicopathological characteristics and results are summarized in Table 1.
The mean age was 67 years (range 38-85), with a male to female ratio of 9:6. The mean larger diameter of the resection piece was 140.9 mm (range 105-270) and lesion was 125.9 mm (87-238), with 53.3% (n = 8) involving ≥75% of the lumen circumference, and 13.3% (n = 2) ≥90%. Lesions were located in >1 rectal segment (n = 12, 80.0%) and distal rectum (n = 3, 20.0%), with 60% (n = 9) involving the pectinate line. Macroscopic morphology demonstrated 11 LST-G (73.3%, 1 homogenous, and 10 nodular mixed) and 4 LST-NG (26.7%). In virtual chromoendoscopy, lesions were JNET 2B in 73.3% and JNET 2A in 26.7%. All lesions were naive to previous therapeutics. Figure 1 illustrates the procedure related to one of the nodular-mixed LST-G resections.
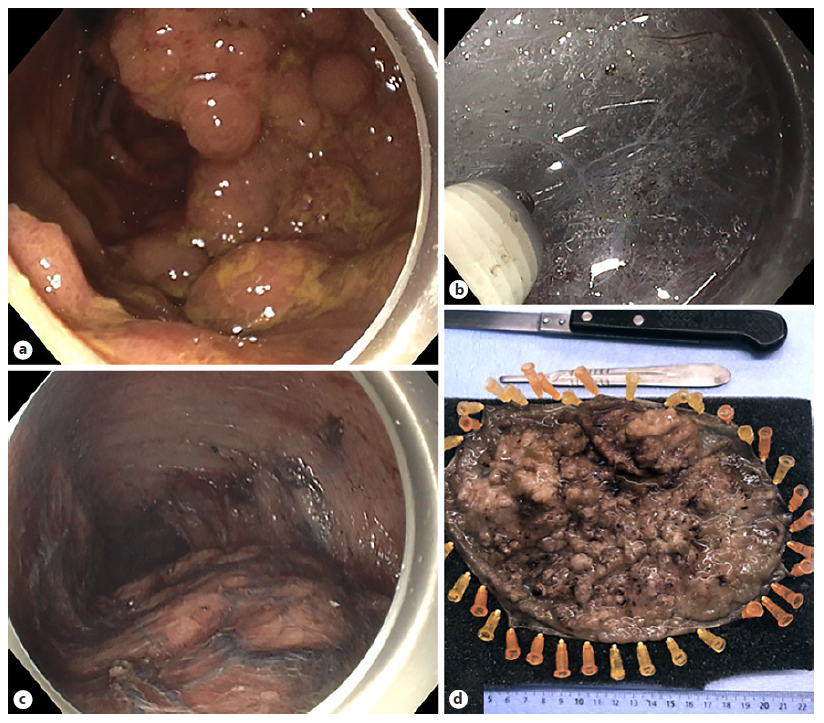
Fig. 1 Resection of a rectal nodular mixed LST-G. a Endoscopic appearance of a giant rectal lesion. b Submucosal dissection using a non-insulated tip knife. c Advanced phase of the procedure where the lesion lies below and the mucosal defect above. d Resection piece after formalin fixation.
The mean procedural time was 220 min (range 80-540 min) and mean post-procedural hospital stay was 2 days (range 1-5 days). En bloc resection was achieved in 100% (n = 15). Procedural complications occurred in 4 patients (26.7%), being discriminated as intraprocedural micro-perforation in 6.7% (n = 1) and delayed local stenosis in 20.0% (n = 3). The perforation was successfully closed by endoscopic clipping. No bleeding complications were observed, early or delayed. Regarding stenosis, all lesions involved the pectinate line and ≥75% of the lumen’s circumference (2 of them involved ≥90% of the circumference). Of these patients, one was submitted to surgery because of concomitant high-risk non-curative resection, and the other two were submitted to endoscopic dilation, with favorable clinical response. Fibrosis and muscle retracting sign were observed in 33.3% of cases (n = 5). None of the procedures was interrupted because of complications nor was converted into surgery.
Pathologically, there were 3 submucosal carcinomas (20.0%), with a mean of 1,617 micromillimeters of deep invasion. The remainder of the lesions were adenomas: 7 tubulovillous (46.7%), 3 sessile serrated (20.0%), 1 tubular (6.7%), and 1 villous (6.7%), divided into 9 with high-grade (75.0%) and 3 with low-grade (25.0%) dysplasia.
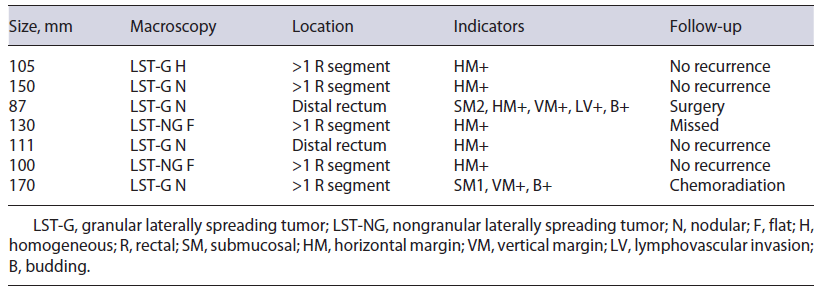
On histology, R0 resection was achieved in 8 patients (53.3%). Patients who did not achieve R0 included 5 (33.3%) with only positive horizontal margins (RX), 1 (6.7%) with only positive vertical margin (R1), and 1 (6.7%) with both margins positive (R1). Budding and/or lymphovascular invasion were noted in 2 (13.3%) patients. According to the ESGE criteria, resections were considered curative in 8 patients (53.3%), non-curative with high risk in 2 (13.3%), and local risk in 5 (33.3%). In the latter, 60% (n = 3) involved the pectinate line. Characterization of non-curative resections is summarized in Table 2.
Additional treatments (chemoradiation or surgery) were indicated in 2 patients (13.3%): 1 had been submitted to surgery and 1 had undergone chemoradiation, with no recurrence detected. For the other patients, endoscopic follow-up was recommended (3-6 months for RX and 1 year for R0). The mean follow-up interval was 16.0 months, where 76.9% (n = 10) respected the recommended surveillance, with no recurrence identified (rate, 0%).
Statistical Analysis
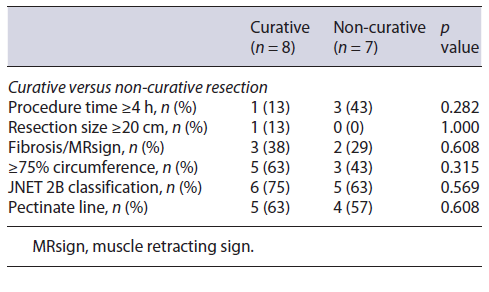
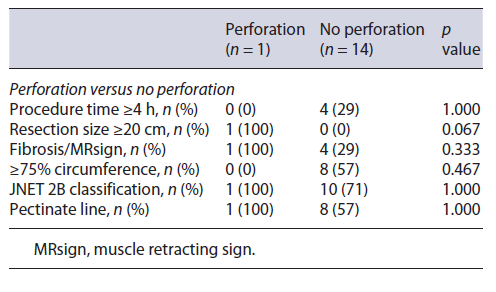
Regarding curative rates and complications (perforation and stenosis), univariate analysis through Fisher’s exact test in SPSS was applied in order to identify significant risk factors for these outcomes. Analysis revealed resection size ≥20 cm as significantly associated with perforation (p value 0.067) and involvement of ≥90% of the lumen’s circumference and procedural time ≥4 h as significantly associated with stenosis (p value 0.029 and 0.009, respectively). No other factors were found to be significantly associated with the rate of complications or curative status, such as procedure time, the presence of fibrosis, JNET classification, and the involvement of the pectinate line (shown in Tables 3-5).
Discussion
ESD is a technically difficult procedure, available worldwide, used for the treatment of early gastrointesti-nal cancers or precancerous lesions. In this study, our ESD outcomes for giant gastrointestinal lesions have been reported. The en bloc and the curative resection rates were 100.0% and 53.3%, respectively, and the adverse event rate was 26.7%, mostly managed endoscopically. Resections with risk of local recurrence (RX) accounted for 33.3%, with 0% of recurrence rate during a mean of 16 months of follow-up. Previous studies revealed better rates; however, most are not comparable as they are not related to giant lesions. In one study (n = 9), the en bloc and curative resection rates of ESD for giant colorectal LST were 88.9% and 100%, respectively, with a higher rate of adverse events (44.4%) [2]. In another study (n = 10) of ESD for giant colorectal LST, the en bloc and curative resection rates were 100% and 90%, respectively, with 40% of adverse events [5]. As shown in Table 2, ESD was considered non-curative with high-risk or local-risk recurrence because of positive horizontal and/or vertical resection margins, submucosal invasion, lymphovascular involvement, or budding. During follow-up of these high-risk non-curative resections, 1 was submitted to surgery and 1 underwent chemoradiation, without any complications or recurrence. Of the patients with significant local recurrence risk, most respected follow-up, and none suffered disease recurrence until the time of the study. However, most follow-up times were less than 2 years.
As for factors affecting the curative resection rate, our analysis was not able to show any significant difference regarding factors such as procedure time, tumor size, the presence of fibrosis, or JNET evaluation (Table 3). This is not concordant with previous studies that correlated tumor size, submucosal fibrosis, invasive depth, and procedure time with incomplete resection [4]. Differences might be related to the small number of cases in our study and to our sample’s universe being filtered to >10 cm lesions, which is rare and innovative and, therefore, not comparable with results from series of all kinds of ESD.
Regarding complications, our sample analysis revealed significant association between resection size and higher risk of perforation (Table 4). This is concordant with pre-vious studies. A user-friendly risk score model for the prediction of risk of perforation has already been delineated, named SELF, which includes factors such as tumor size, endoscopist’s experience, tumor location, and submucosal fibrosis [6]. In our study, location was not a variable, as all lesions were located in the rectum, the safest site for ESD because of its thick wall and fixed position. There was 1 microperforation, treated endoscopically without the need for additional surgery.
Regarding stenosis, our analysis showed a significant association between involvement of ≥90% of the circumference and a higher risk of stenosis (Table 5). This result is concordant with previous literature, where in different gastrointestinal locations, lesions spreading to ≥75% and ≥90% of circumference were associated with higher stenosis rates [7]. Other studies have also associated the involvement of the dentate line with higher rates of postoperative pain and strictures, which was not significant in our sample [8]. Our patients had received some kind of stenosis prophylaxis (corticosteroids), which is still controversial and currently not recommended. From the 3 stenoses, 1 was operated on since the patient had concomitant high-risk non-curative resection, and the other 2 were submitted to endoscopic dilation with favorable responses. None had incapacitating symptoms. Regaring the association between procedural time and stenosis, it might be explained by the dependency of this variable on others (such as size, circumference involvement). Previous studies have only related procedure time to post-ESD coagulation syndrome [9].
As for bleeding complications, none were reported in our cohort, which might be explained by the routine prophylactic coagulation of visible vessels. There were several limitations to this study. First, we highlight the small sample size, one endoscopist, single-centered, and retrospective nature of the study. Second, no comparison was made with surgical outcomes of giant lesions or between groups of LSTs ≥10 cm versus <10 cm. A multicentered study with several endoscopists, higher sample and follow-up time is recommended to achieve more significant and generalizable results and outcomes. Variants of ESD with tunneling and pocket-creation methods might also be studied [10].
Moreover, according to our previous studies, a minimum of 30 procedures must be carried out to significantly increase en bloc resection, and 90 procedures are needed to achieve a R0 resection rate >75%, with concomitant speed improvement. Indeed, we suggest that, ideally, only experienced endoscopists (preferably with >90 previous procedures) should carry out giant resections [11, 12].
In conclusion, ESD is an important novel organ-sparing therapeutic endoscopy for the treatment of early gastrointestinal neoplasia. Once the learning curve for ESD is overcome, previous studies have demonstrated that this technique is safe, effective, and worth pursuing.