Introduction
Stroke represents the second cause of death in the world 1 and the second cause of years of healthy life lost due to disability 2. One of its more frequent complications is dysphagia, which can reach 80% in the acute phase 3. There is an unequivocal relation between dysphagia and respiratory complications 4 as well as increased risk of dehydration 5 and malnutrition 6. These complications result in an increased length of stay, institutionalization, mortality and poorer functional outcomes. In addition to the impact on the patient, it also has a great impact on health costs, with an increase of 40.36% 7.
Several clinical guidelines list in a dispersed way recommendations for assessment and management of dysphagia after stroke, making it difficult to translate evidence to clinical practice. Therefore, it is essential to develop specific clinical guidelines that support good practice for dysphagia management in stroke patients, from onset to rehabilitation. The framing, implementation and utilization of clinical guidelines, grounded on the best available evidence and adjusted to clinical contexts, guarantee the quality and excellence of care provided to stroke patients.
The objective of this work is to provide health professionals with a set of clinical recommendations for the therapeutic approach to stroke patients with dysphagia to streamline the evidence-based practice.
Methods
The World Health Organization 8 guidelines for the development of clinical practice recommendations were used. A preliminary literature search allowed to narrow and define accurately the areas which researchers aimed to cover with the recommendations. Experts were also consulted to clarify and focus research on the scope of practice. Three investigators defined the areas that the set of recommendations should cover, as well as the target population (stroke patients) and clinical condition (dysphagia), from acute to rehabilitation stage, covering diagnostic and therapeutic evidence-based interventions that decrease morbidity and mortality or improve outcomes. From there, seven clinical questions were formulated in PICO format.
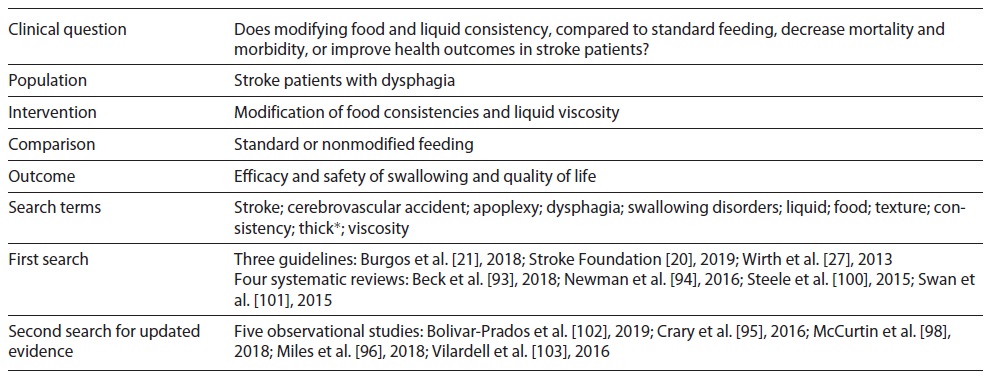
Then, the methodology for a systematic literature review was defined, which included the search for clinical guidelines, systematic literature reviews/meta-analysis and experimental or quasi-experimental studies and observational studies, published between 2008 and 2019 and in Portuguese, English and Spanish, in the following databases: Cochrane Library; CINAHL with Full Text; PubMed complemented by manual search on pages of scientific/professional societies in related areas and entities responsible for issuing clinical guidelines. Database search was conducted during 2019. Due to the complexity of the literature search, an example of the search strategy is shown in Table 1 and the full search strategy is available in the supplementary material (for all online suppl. material, see https://www.karger.com/doi/10.1159/000520505).
The clinical guidelines found were analyzed to identify relevant content and selected accordingly. Then, systematic reviews and meta-analyses were searched. The search for experimental/quasi-experimental or observational studies was dependent on the recovered reviews and the evaluation of its methodological quality. Depending on the publication date, the search was repeated for the subsequent period, in an attempt to identify studies published for further analysis.
Regarding the assessment of methodological quality, it was carried out by two independent researchers. AGREE II was used for the assessment of clinical guidelines 9, and those that obtained an average score in all domains ≥70% were considered for inclusion. The assessment of the methodological quality of systematic reviews was guided by AMSTAR 2 10 and moderate to high-quality reviews were included. For primary research, the tools provided by the Critical Appraisals Skills Program were used 11.
For the preparation of the first draft of recommendations, clinical guidelines and/or systematic reviews of high methodological quality were used directly, as long as they were updated. The existence of high quality and/or relevant experimental or quasi-experimental studies published after the publication of clinical guidelines determined their analysis and inclusion for discussion by experts. At the end of this phase, the level of evidence (LOE) was rated according to Halperin et al. 12. LOE level A is determined by high-quality evidence from more than one randomized controlled trial (RCT), meta-analyses of high-quality RCTs or one or more RCTs corroborated by high-quality registry studies; LOE level B-R is from moderate-quality evidence from one or more RCTs or meta-analyses of moderate quality RCTs; LOE level B-NR emerges from moderate-quality evidence from one or more well-designed, well-executed nonrandomized studies, observational studies or registry studies or meta-analyses of such studies; LOE level C-LD by randomized or nonrandomized observational or registry studies with limitations of design or execution or meta-analyses of such studies or physiological or mechanistic studies in human subjects, and LOE level C-EO is supported on a consensus of expert opinion based on clinical experience 12. After this process, a first draft was elaborated with a set of 23 recommendations for submission to a panel of experts in a Delphi-type technique by e-mail. This technique was intended to develop an expert-based judgment, based on the assumption that a group of experts and a variety of perspectives will produce a more valid result than just an individual. Each expert was asked to indicate the level of agreement for each recommendation (agree, partially agree or disagree), and for the responses “partially agree” and “disagree” they were asked to justify their answer on clinical/scientific grounds. The consensus was defined for levels of agreement above 80%. Regarding the recommendation level, which concerns the magnitude of the benefit over the risk, an account was taken of the LOE existing for each recommendation and the degree of agreement and justification presented by each of the experts. The rating was performed according to Halperin et al. 12: class of recommendation (COR) I (strong), when the treatment/procedure/intervention is useful and effective and should be provided to most patients under most circumstances; COR IIa (moderate), lower benefit over risk than COR I; COR IIb (weak), where the benefit is marginally higher than the risk; COR III, when the treatment/procedure/intervention is not recommended 12. COR and LOE were assessed independently. Halperin et al. 12 argue that any COR can be paired with any LOE. A LOE of limited data does not imply that the recommendation is weak. On one hand, experts may consider that, based on their clinical experience, the clinical benefit of an intervention may be evident. On the other hand, a specific intervention may not be suitable to be tested in a randomized controlled trial, making evidence weak, conflicting or absent in which cases authors advocate that guidance is foremost needed 12. Therefore, the classification of the recommendation was based on the available evidence and its clinical relevance. It was also defined that only levels of agreement above 90% would allow classification up to class I (strong) and that levels of agreement above 80% but ≤90% would only have, at most, class IIa classification (moderate).
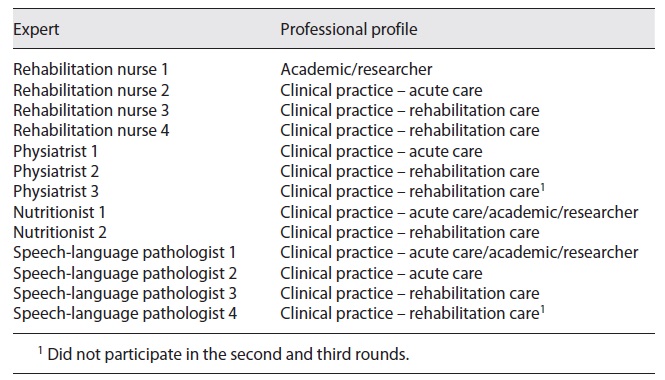
Recognizing that the Delphi technique is not a research method aligned with any specific methodology, the difficulty in defining quality criteria for its development that are widely accepted is highlighted 13. In this sense, and to reduce the risk of habitus mentalis, experts from different professional areas, from different contexts of practice, both clinical and academic, were invited to participate in the purposive sampling technique. Diversity in a group of experts favors a more innovative discussion, thus becoming as relevant to the final result as the skills and expertise of the participants, enhancing the robustness and validity of the findings. Regarding the number of experts to invite, the available evidence is not consensual, with the number of experts varying from 3 to 713, with an average of 14 13. For the Delphi panel of this study, 16 more easily accessible experts who agreed to participate were invited: 4 specialist nurses in rehabilitation nursing, 1 neurologist, 5 physiatrists, 2 nutritionists and 4 speech-language pathologists (SLPs). All experts had more than 10 years of effective clinical and/or academic experience in the field of stroke. All experts were previously contacted to clarify the type and terms of their participation. Regarding ethical considerations related to the Delphi panel, the experts were also asked to declare any type of conflict of interest. The experts had no declared affiliation that could be perceived as posing a potential conflict of interest in the participation in this Delphi panel. The anonymity of the participants was also ensured. No patients were involved in the experts’ panel.
Results
The systematic review of the literature and manual research resulted in a first draft of 23 recommendations. None of the guidelines already published answered all the clinical questions initially formulated. The draft with the 23 recommendations was then submitted to the expert’s appraisal. Of the 16 invited experts, 13 responded: 4 rehabilitation nurses, 3 physiatrists, 2 nutritionists and 4 SLPs (Table 2).
In the first round, a consensus was obtained for 14 recommendations and of these, 9 reached a consensus of over 90%. Recommendations that reached a consensus level (in the sum of total and partial consensus) above 80%, but below 90%, went on to the next round with the incorporation of the experts’ suggestions. Three recommendations reached consensus levels (in the sum of total and partial consensus) below 80% and were therefore rejected. For the second round, 7 recommendations were sent to the experts and an additional one emerged from the expert’s opinion. To clarify some of the issues pointed at by the experts in the previous round, additional information was sent explaining the rationale for decision-making. In this round, all recommendations reached a level of consensus higher than 80%.
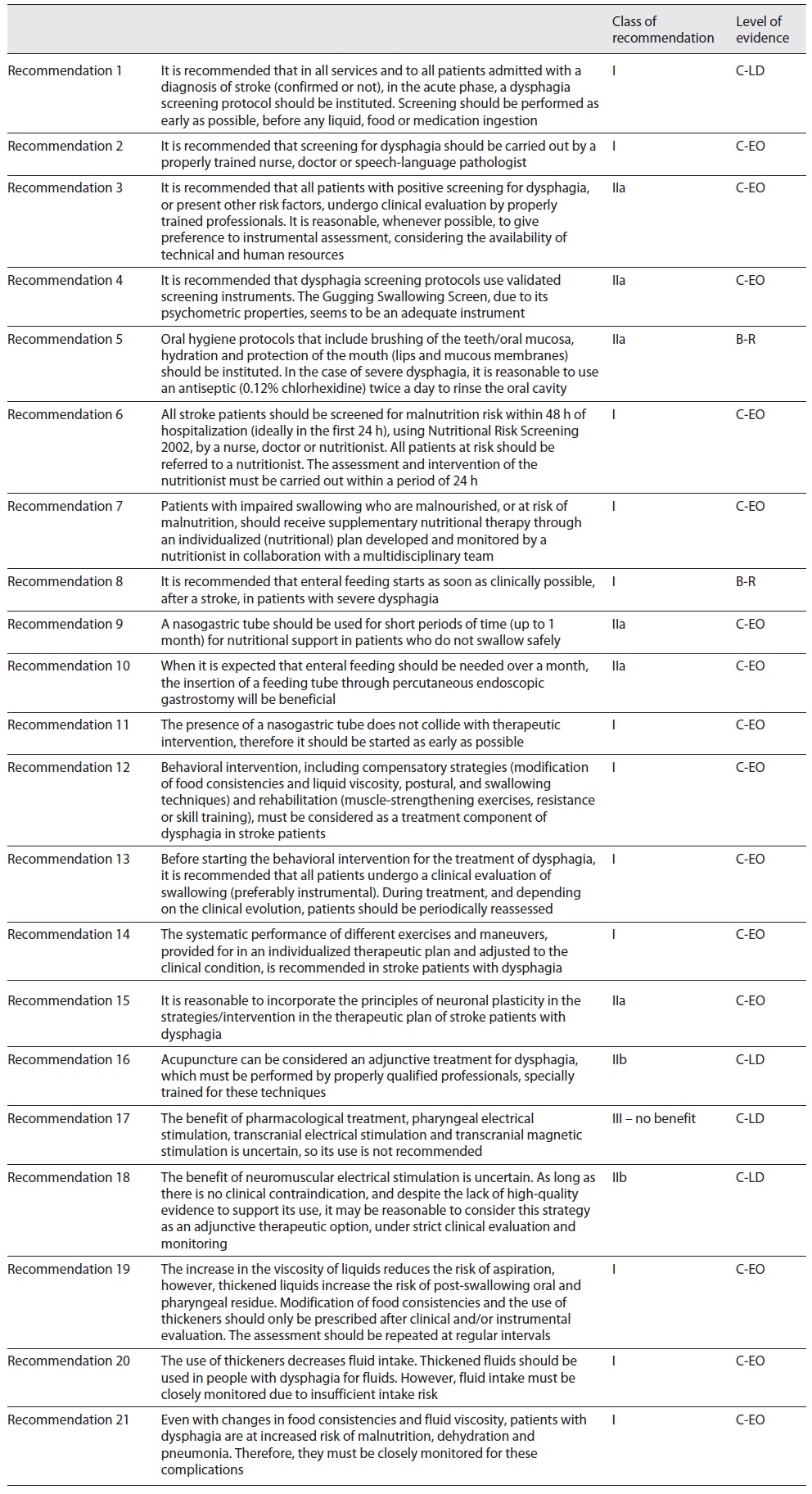
The final version of the recommendations was sent to the experts, with an indication of the level of consensus obtained for each of them and the level of recommendation, for final validation. After the appraisal, a final version with 21 recommendations was obtained (Table 3).
1 Clinical Question
When should the ability to swallow be screened and assessed in stroke patients?
1.1 Recommendation Statement(s)
It is recommended in all services and to all patients admitted with a diagnosis of stroke (confirmed or not), in the acute phase, to implement a dysphagia screening protocol. It is recommended that screening be performed as early as possible, before any liquid, food or medication ingestion.
COR I
LOE C-LD
It is recommended that all patients with positive screening for dysphagia, or present other risk factors, undergo clinical evaluation by properly trained professionals. It is reasonable, whenever possible, to give preference to instrumental assessment, considering the availability of technical and human resources.
COR IIa
LOE C-EO
It is recommended that screening for dysphagia should be carried out by a properly trained nurse, doctor or SLP.
COR I
LOE C-EO
1.2 Summary of Evidence
For dysphagia assessment, more specifically for the identification of aspiration (passage of material to the larynx - food or liquids - below the level of the vocal folds), instrumental evaluation (videofluoroscopy swallowing study) has been considered the gold standard 14. Ideally, all patients should be evaluated with reference tests; however, there are several limitations: not all patients can undergo an invasive examination, nor do all hospitals have trained professionals available 24 h a day to perform them and not all hospitals have the necessary equipment 15. As a result, instrumental assessment is not accessible to all stroke patients in the acute phase. Therefore, dysphagia screening is recommended in several clinical guidelines for all patients admitted with stroke, as early as clinically possible, before the administration of any food, liquid or medication 15), (16), (17), (18), (19), (20), (21), (22. Of all these recommendations, only one sets a time interval of up to 4 h after admission 20. All the others indicate as early as possible and as soon as the patient’s level of consciousness allows it. The supporting evidence results are essentially from observational studies. In fact, a systematic review published in 2018, which sought to identify RCTs that studied the effect of dysphagia screening protocols on the incidence of pneumonia, death or dependence after stroke, only 3 studies were identified, and the results did not determine any effect on these outcomes 23. Another systematic review of observational and quasi-experimental studies, which included 12 studies with more than 87,000 participants, suggests that early screening for dysphagia reduces the incidence of pneumonia after stroke 24. According to the authors, the heterogeneity of the methods prevented the quantitative analysis of the results. Evidence supporting the international recommendations comes essentially from studies included in this latest review 24. An observational study published later and not included in this review 25, with a sample of 3,309 participants, who sought to assess the impact of delay in screening for dysphagia in stroke patients, regarding its performance within 4 h after admission, concluded that the delay is harmful, suggesting that this may be associated with the delay in nutrition and the use of inappropriate feeding techniques, which enhances aspiration. This study reinforces the existing evidence, thus justifying the level of recommendation proposed for the first recommendation. Thus, screening is the first step to identifying patients at risk and in need of a more comprehensive clinical or instrumental assessment 17), (18), (19), (26), (27. Silent aspiration is an added challenge for health professionals, and the range of dysphagia screening tools available does not adequately address this dimension of dysphagia. Most of them consider open signs of aspiration but do not consider, for example, the capacity for reflex coughing, which reflects the degree of damage to the laryngeal sensorimotor response 28), (29, thus reinforcing the need for clinical evaluation not only for patients with positive screening, but also for those with other risk factors. The American Heart Association/American Stroke Association (AHA/ASA) recommend, in a moderate grade, that all patients must undergo instrumental assessment subject to the availability of human and technical resources 16), (22, arguing that instrumental assessment allows visualizing the physiology of swallowing and determine the presence or absence of aspiration, which is necessary for the definition of an adequate therapeutic plan. Other guidelines suggest instrumental assessment following an inconclusive clinical assessment/suggestive of aspiration or in enteral-fed persons 17), (18. Another guideline, recognizing the usefulness of performing clinical assessment, recommends that preference be given to instrumental assessment 21. The recommendation essentially emerges from the awareness of the limited human and technical resources for prioritizing instrumental assessment. Regarding the professional most qualified to perform the screening, part of the international guidelines recommend that it should be performed by an SLP 16), (17), (22 or another properly trained professional, which they do not specify 18), (19. One of the recommendations does not refer to the professional who should perform the screening 21. Evidence suggests that screening by nurses is effective in an important set of outcomes in stroke patients, namely in reducing the incidence of pneumonia 30), (31. In this context, there is a relatively established consensus that screening for dysphagia in stroke patients should be carried out by nurses as soon as possible, so that patients are not kept nil by mouth for an unnecessary time, considering that an SLP is not available 24 h/day in hospitals 31), (32.
1.3 Certainty of Evidence of Effects
Despite a wide international consensus amongst experts for early screening, most of the available data are observational and quasi-experimental studies (limited data) and are not conclusive. Nevertheless, the available evidence suggests higher benefit in screening versus no screening and early screening versus delayed screening. The remaining recommendations stand mostly on experts’ opinions.
1.4 Summary of Delphi Panel’s Results
For the first recommendation, 100% of the total consensus was obtained in the first round. Concerning instrumental assessment, to note that one of the experts stated that it should be clear that only SLPs can perform the clinical assessment and subsequent intervention, adding that the instrumental assessment should only be performed in case of doubtful clinical assessment. Regarding professionals fit to screen stroke patients, one of the experts partially agreed with the recommendation, stating that SLPs should perform clinical assessment and not screening. Screening should be performed by properly trained nurses or physicians. Two other experts suggested including the physician, noting that, in certain clinical contexts, the physician also performs screening. The recommendation built from the experts’ consensus was classified as strong given the need to, following the recommendation regarding early screening, ensure that clear guidance is provided.
1.5 Conclusions and Research Need for this Recommendation
Unequivocal evidence on the impact of early dysphagia screening in acute stroke patient’s outcomes is insufficient. RCTs are needed to determine the effect on reducing pneumonia rates and death and on the improvement of functional outcomes. These findings and suggestions for future trials do not mean that screening should not be performed. Strong consensus in both national and international experts is found on this subject. For dysphagia, more specifically for the identification of aspiration, instrumental assessment has been considered the gold standard. However, it is essential to further alignment in defining the focus of research on the assessment of dysphagia, with significance for clinical practice. Limited resources for the realization of instrumental assessment could even be exceeded in the context of clinical trials through funding, but it would not mimic reality in clinical settings, limiting the applicability of these results.
2 Clinical Question
What tools are available to screen dysphagia in acute stroke patients?
2.1 Recommendation Statement
It is recommended that dysphagia screening protocols use validated screening instruments. The Gugging Swallowing Screen (GUSS), due to its psychometric properties, seems to be an adequate instrument.
COR IIa
LOE C-EO
2.2 Summary of Evidence
There is a consensus on the need to implement dysphagia screening protocols in acute stroke patients; however, not all stroke units use dysphagia screening protocols, and in Portugal, there is evidence that nurses use water tests and different items for informal dysphagia screening 33. Informal detection, despite having high specificity, has low sensitivity, which means that a significant percentage of dysphagic patients will not be identified, translating into an increased risk of complications 34. Water tests and pulse oximetry assessment, traditionally used to identify aspiration, are often used as dysphagia screening strategies, whereas for water tests, the result for sensitivity is less than 80% 35 and concerning pulse oximetry, the existing evidence cannot prove the existence of an association between oxygen desaturation and aspiration, thus not validating its use in detecting aspiration 36. These findings reinforce the need to implement dysphagia screening protocols to identify patients at risk who need subsequent clinical evaluation 33. For screening, no specific tool has been recommended so far in most clinical guidelines probably due to the difficulty in developing instruments with the ideal psychometric properties, i.e., adequate sensitivity, specificity and predictive strength that allow to detect dysphagia and aspiration when used by any health care professional 17), (19), (20), (26. A systematic review identified three dysphagia screening tools for stroke patients, validated against the gold standard 37. Given its psychometric properties and dietary recommendations, the GUSS seems to be the most appropriate tool available so far 37 and is referred to in two clinical guidelines 18), (27. It is a two-part screen, comprising in the first part the test of indirect swallowing items (level of consciousness, saliva swallowing, voice changes, drooling and voluntary cough) that, if successfully achieved, leads to the part where swallowing items are tested with different consistencies and volumes of liquids, semisolid and solid textures, in a series of sequential subtests 38.
2.3 Certainty of Evidence of Effects
Evidence on the use of the GUSS is increasing, and a systematic review 39 showed in a pooled analysis a sensitivity of 0.97 and specificity of 0.67, concluding that the screening performed by nurses using the GUSS reduces the time for screening and the incidence of pneumonia making it a valid and sensitive tool for dysphagia screening. Thus, it is expected that the benefit of using a dysphagia screening tool in patients with stroke is higher than not using one.
2.4 Summary of Delphi Panel’s Results
A very strong consensus was obtained for this recommendation. Two experts stated some reservations about the use of the GUSS, noting that the instrument has some gaps that would be important to fill, adding that from all of the available screening tools, this one seems to be the most suitable.
2.5 Conclusions and Research Need for this Recommendation
A single screening tool may not be adequate to all clinical settings due to organizational, clinical or structural issues and availability of resources, that is why concerns in research should focus on substantiating the effectiveness of the tools already developed, to strengthen the results of validity and reliability, as well as assess the impact on outcomes in stroke patients. Different tools have been developed with similar psychometric properties; spending resources on research to develop new screening tools does not seem to bring better outcomes for patients.
3 Clinical Question
Does the introduction of oral hygiene protocols, compared to standard oral hygiene care, decrease mortality and morbidity or improve health outcomes in stroke patients with dysphagia?
3.1 Recommendation Statement
It is recommended to implement oral hygiene protocols that include brushing of the teeth/oral mucosa, hydration and protection of the mouth (lips and mucous membranes). In the case of severe dysphagia, it is reasonable to use an antiseptic (0.12% chlorhexidine) twice a day to rinse the oral cavity.
COR IIa
LOE B-R
3.2 Summary of Evidence
Stroke patients are at increased risk of oral colonization by respiratory pathogens, which is associated with adverse respiratory events 40), (41 and poorer quality of life 42. Furthermore, they have a worse clinical oral health status in several parameters: tooth loss, number of caries, periodontal status and oral hygiene 43), (44. On the other hand, there is also evidence of the association between dysphagia and an increased risk of pneumonia 45), (46 which, in turn, is associated with an increased time of hospitalization, worse functional outcomes and increased risk of mortality 47. Despite this, the available evidence does not allow for an independent association between dysphagia and aspiration pneumonia, as it is multifactorial. There is evidence to suggest that patients with a nasogastric tube (NGT), whose presence promotes colonization of the oropharynx, patients with severe mobility impairment and patients with an altered state of consciousness are at greater risk of developing aspiration pneumonia 4), (48. On the other hand, a study developed by Kalra et al. 49 concludes that the early use of an NGT in stroke patients not orally fed does not increase the incidence of pneumonia, mortality or worse functional outcomes. In addition, free water intake, under specific conditions, in patients with dysphagia after stroke, does not increase the incidence of aspiration pneumonia, thus reinforcing its multifactorial etiology 50.
In this context, international guidelines emphasize the importance of implementing oral hygiene programs, that show explicitly the frequency of mouth care, devices and products to be used, stressing the importance of these protocols for pneumonia reduction 16), (17), (18), (19), (20), (22. Oral care after every meal and bedtime is recommended 19, or twice a day 51), (52, and it is considered reasonable to perform it with a rinse with chlorhexidine to reduce the risk of pneumonia, dental plaque and bleeding gums 16), (20), (51), (52.
The use of chlorhexidine in a rinse solution or gel in oral hygiene care has been shown to be effective in reducing ventilator-associated pneumonia in critically ill patients 53; however, there is insufficient evidence to establish this relationship in stroke patients. Updated evidence shows that adding chlorhexidine to oral care reduced dental plaque and gingival bleeding 54, and the incidence of aspiration pneumonia 55. The implementation of oral hygiene programs with increased frequency and intensity suggests a significant improvement in oral health 56, and its implementation associated with the use of other oral solutions for washing and rinsing also suggests improved oral health and reduced incidence of aspiration pneumonia 57.
The need for training and education for health professionals on the assessment, provision and importance of oral care in preventing pneumonia is acknowledged 20. The intervention of professionals in oral hygiene seems to improve the knowledge and attitudes of professionals and reduce the incidence of pneumonia 58. Despite that, oral care is overlooked in clinical practice which reflects the professionals’ lack of knowledge in oral care. Contributing to it is the lack of evidence and specific guidelines for stroke patients.
3.3 Certainty of Evidence of Effects
Evidence suggests that a superior benefit resulting from the implementation of oral hygiene protocols that include brushing of the teeth/oral mucosa, hydration and protection of the mouth (lips and mucous membranes) in stroke patients will be expected compared to its nonimplementation 59), (60 and that the use 0.12% chlorhexidine has a favorable effect on the incidence of respiratory complications in patients with severe dysphagia 55.
3.4 Summary of Delphi Panel’s Results
A strong consensus was obtained for this recommendation. One of the experts partially agreed on this recommendation stating that the recommendation should include the frequency of oral care. Another expert raised some concerns on the effect of chlorhexidine on the swallowing ability, justifying a partial agreement to the recommendation. For this reason, further search was made of RCTs on the effect of chlorhexidine on swallowing ability, with no results. The most frequently reported adverse effects reported are changes in taste, changes in the oral mucosa (discomfort/pain, irritation, slight desquamation and ulceration/erosion) and burning sensation in the mouth and/or tongue 61.
3.5 Conclusions and Research Need for this Recommendation
Systematic oral care may reduce the incidence of pneumonia; however, further studies to make this relationship unambiguous are needed 62. The analysis of the evidence found highlights the need to produce evidence, with more robust designs, to identify the oral hygiene protocol that produces the best results improving oral health, quality of life and reducing the incidence of complications.
The need for education and training on oral care for health professionals, especially nurses, and caregivers is emphasized.
4 Clinical Question
Does systematic screening for nutritional risk, compared to standard care, in stroke patients with dysphagia decrease mortality and morbidity or improve health outcomes in stroke patients?
4.1 Recommendation Statement(s)
It is recommended to screen all stroke patients for malnutrition risk within 48 h of hospitalization (ideally in the first 24 h), using Nutritional Risk Screening 2002 (NRS 2002), by a nurse, doctor or nutritionist. It is recommended to refer patients at risk to a nutritionist. It is recommended that the assessment and intervention of the nutritionist be carried out within a period of 24 h.
COR I
LOE C-EO
It is recommended that patients with impaired swallowing who are malnourished or at risk of malnutrition receive supplementary nutritional therapy through an individualized (nutritional) plan developed and monitored by a nutritionist in collaboration with the multidisciplinary team.
COR I
LOE C-EO
4.2 Summary of Evidence
There is evidence of an association between dysphagia and increased dehydration in the first 7 days after stroke 5, as well as an increase in malnutrition 5), (6. These complications result in increased length of stay, greater likelihood of institutionalization after discharge, increased mortality and worse functional outcomes 7), (45), (63. Furthermore, the nutritional deficit is also associated with a worse quality of life in stroke patients 64. It is in this context that different international guidelines recommend nutritional risk assessment to all patients after a stroke, however with differences in recommendations. The European Society for Clinical Nutrition and Metabolism (ESPEN) 21 recommends the use of the Malnutrition Universal Screening Toll (MUST) based on the validation study of MUST for stroke patients 65. In Portugal, guidelines recommend the use of Nutritional Risk Screening 2002 (NRS 2002) 66.
Recognizing the increased risk of malnutrition in stroke patients with dysphagia, nutritional risk assessment is recommended to all patients within the first 48 h of hospitalization (ideally during the first 24 h), using the NRS 2002 66. Screening can be performed by a nurse, a doctor or a nutritionist, and patients at risk should be referred for nutritional assessment and intervention within 72 h after admission 66. Patients must be weekly reassessed 20 and for patients at nutritional risk, individualized nutritional support is recommended 67.
4.3 Certainty of Evidence of Effects
Nutritional support does not reduce adverse events or show any effect in reducing mortality 67; however, the recommendation level is strong, supported essentially by expert opinion, considering that the benefit of the intervention greatly outweighs the risk.
4.4 Summary of Delphi Panel’s Results
The first version of the recommendations on systematic nutritional screening proposed the MUST as a screening tool, based on the ESPEN recommendations, a proposal that was rejected by several experts, referring to national guidelines. The recommendation was then revised, and in a second round received full agreement from all experts.
4.5 Conclusions and Research Need for this Recommendation
The difference in the screening tool’s recommendation may be related to the fact that national guidelines are not specifically aimed at stroke patients. Nevertheless, wide consensus exists on the need to screen stroke patients and provide individualized nutritional therapy developed and monitored by a nutritionist. Research is needed to determine the effects of nutrition support on death, dependency and other health-related outcomes.
5 Clinical Question
Does NGT feeding in patients with severe dysphagia, compared to other enteric feeding strategies, decrease mortality and morbidity or improve health outcomes in stroke patients?
5.1 Recommendation Statement(s)
It is recommended that enteral feeding starts as soon as clinically possible, after a stroke, in patients with severe dysphagia.
COR I
LOE B-R
NGT can be useful for short periods (up to 1 month) for nutritional support in patients who do not swallow safely.
COR IIa
LOE C-EO
When it is expected that enteral feeding should be needed over a month, the insertion of a feeding tube through percutaneous endoscopic gastrostomy (PEG) can be beneficial.
COR IIa
LOE C-EO
The presence of NGT does not collide with therapeutic intervention, therefore it is recommended to start as early as possible.
COR I
LOE C-EO
5.2 Summary of Evidence
Malnutrition in stroke patients can have a severe impact as it enhances worse functional results and worse quality of life 68, putting at risk the rehabilitation process. Malnutrition risk is also an independent predictor of mortality, longer hospital stay and hospitalization costs 65. Enteric feeding is, in situations of severe dysphagia, the only way to ensure nutritional and hydration intake, as well as allowing the administration of medication 69. However, its use is not without risks, with mechanical complications, such as nasal trauma, displacement, poor positioning and obstruction 70, gastrointestinal complications 71, metabolic disorders 72, resulting in a worse quality of life 73. Different international guidelines recommend as soon as possible enteral nutrition by NGT in patients who, after a stroke, do not swallow safely, with recommendations varying between the first 24 h 18, the first 3 days 17), (19), (21 or the first 7 days 16), (22), (27. The Australian recommendations only indicate, with a weak level of recommendation, that NGT enteral feeding is the preferred short-term method of feeding stroke patients 20.
The early placement of an NGT for feeding is justified by the enhanced nutritional risk, thus allowing adequate nutrition and hydration 21. Wirth et al. 27 alert that these patients have a higher risk of aspiration and aspiration pneumonia that is not prevented with the placement of an NGT, so the risk of aspiration cannot be the reason for its placement. It should be noted that the evidence regarding the increased risk of pneumonia associated with NGT placement is contradictory 74.
Procedures for verifying the correct positioning and fixation of the NGT are recommended, considering the risks of displacement and accidental removal, issues with a significant impact on nutrient supply and a common problem in clinical practice 21. Therefore, the use of small caliber tubes (8 CH) is recommended to minimize the risk of ulceration; the verification of the correct placement of the NGT should be performed through Rx or aspiration of gastric contents for pH measurement and, for fixation, the use of a nasal loop/bridle is advised 21), (27.
If it is expected that the patient will not be able to restart oral feeding for a period longer than 2-4 weeks, it is recommended to place a PEG 14), (16), (21), (22. In the decision to place a tube for enteral feeding by gastrostomy, in stroke patients with an unfavorable prognosis, ethical issues and early manifestation of a will must be specially attended, and, when in doubt, enteric feeding by NGT should be considered as the most appropriate, given the reversibility of the procedure 21.
Therapeutic intervention for dysphagia should start as early as possible, considering that the presence of an NGT does not aggravate dysphagia 75 and does not interfere with swallowing training 27. In fact, the presence of an NGT interferes with the movement of the hyoid bone during swallowing, but that this movement is re-established after removal of the tube 76.
5.3 Certainty of Evidence of Effects
The Feed or Ordinary Diet (FOOD) trial was a well-designed multicentric RCT that aimed to evaluate feeding policies in stroke patients, that was developed in 131 hospitals in 18 countries, with 5,033 stroke patients 77 and is the supporting evidence of most international guidelines. More recent evidence suggests that in the comparison between enteral feeding by NGT versus PEG, PEG is associated with fewer treatment failures, lower incidence of gastrointestinal bleeding, higher feeding delivery and albumin concentration 78. No differences in death or dependency were found.
5.4 Summary of Delphi Panel’s Results
The consultation of the experts revealed high levels of agreement, although it was necessary to reformulate two of the recommendations. The first regarding the time to start enteral feeding (the first draft had a time limit of 72 h), with 72 h being considered an excessively long period by five of the experts. It was agreed that it should be started as soon as clinically possible. The second, regarding the start of therapeutic intervention, in which five of the experts did not agree with the initial wording that stated that the presence of an NGT does not worsen dysphagia, agreeing, however, that the therapeutic intervention should be started as early as possible. For this reason, recommendations were revised to incorporate the experts’ suggestions.
5.5 Conclusions and Research Need for this Recommendation
Despite the lack of robust evidence, adequate nutrition and hydration must be ensured in stroke patients with severe dysphagia considering the nefarious impact of dehydration and malnutrition on the rehabilitation process. This is an area that needs further investigation, recognizing the ethical and logistical challenges that studies with vulnerable populations represent, which translates into the difficulty of conducting truly randomized clinical trials. The possibility of predicting the duration of dysphagia is crucial for the decision-making process regarding the route for feeding. Research must address this issue.
6 Clinical Question
Which therapeutic interventions have the most significant results in the recovery of swallowing function and airway safety?
6.1 Recommendation Statement(s)
It is recommended to consider behavioral intervention, including compensatory strategies (modification of food consistencies and liquid viscosity, postural and swallowing techniques) and rehabilitation (muscle-strengthening exercises, resistance or skills training), as a treatment component of dysphagia in stroke patients.
COR I
LOE C-EO
Before starting the behavioral intervention for the treatment of dysphagia, it is recommended that all patients undergo a clinical evaluation of swallowing (preferably instrumental). During treatment, and depending on the clinical evolution, it is recommended to periodically reassess patients.
COR I
LOE C-EO
The systematic performance of different exercises and maneuvers, provided for in an individualized therapeutic plan and adjusted to the clinical condition, is recommended in stroke patients with dysphagia.
COR I
LOE C-EO
It is reasonable to incorporate the principles of neuronal plasticity in the strategies/interventions in the therapeutic plan of stroke patients with dysphagia.
COR IIa
LOE C-EO
Acupuncture may be considered an adjunctive treatment for dysphagia, which must be performed by properly qualified professionals, specially trained for these techniques.
COR IIb
LOE C-LD
The benefit of pharmacological treatment, pharyngeal electrical stimulation, transcranial electrical stimulation and transcranial magnetic stimulation is uncertain, so its use is not recommended.
COR III - no benefit
LOE C-LD
The benefit of neuromuscular electrical stimulation is uncertain. As long as there is no clinical contraindication, and despite the lack of high-quality evidence to support its use, it may be reasonable to consider this strategy as an adjunctive therapeutic option, under strict clinical evaluation and monitoring.
COR IIb
LOE C-LD
6.2 Summary of Evidence
Defining recommendations for the therapeutic approach to patients with dysphagia after stroke is, most likely, one of the biggest challenges in this context. The range of interventions available is vast and includes behavioral interventions that comprise compensatory strategies (postural and swallowing techniques and dietary modifications) and rehabilitation strategies (strengthening, resistance and skill training) 79. In addition to these interventions, there are other rehabilitation strategies, such as noninvasive neurostimulation techniques: repetitive transcranial magnetic stimulation (RTMS), transcranial direct current stimulation (TDCS), neuromuscular electrical stimulation (NMES) and pharyngeal electrical stimulation (PES) 80; a physical stimulation (tactile, thermal and sour); pharmacological strategies 79 and acupuncture 81. The distinction between the different compensatory and rehabilitation strategies becomes particularly relevant as each of them has specific objectives that are not mutually exclusive: the compensatory strategies aim to ensure adequate and safe hydration and nutrition, preventing complications, and rehabilitation strategies have as main objective the recovery of the swallowing function 14.
The analysis of international clinical guidelines reflects the difficulty to summarize the evidence, including contradictory recommendations regarding the same therapeutic strategy. As an example, the clinical guidelines of the AHA/ASA recommend the nonuse of NMES because its benefits are uncertain, supporting this recommendation in the systematic review produced by the Cochrane Collaboration 78. On the other hand, ESPEN’s recommendations are to recommend its use, alone or, preferably, with behavioral intervention 21. It is important to note that, in addition to these two guidelines, only in the Australian clinical guidelines have recommendations on the use of NMES, recommending its use only by experienced clinicians, been applied according to parameters established in the research context and with a weak level of recommendation 20. Both the ESPEN recommendations 21 and the Stroke Foundation 20 recommendations support its recommendation in a systematic review published in 2016 82 which suggests that, despite the limited number of available studies, NMES appears to be more effective in the short term in post-stroke dysphagia. However, it also states that the existing evidence does not allow to determine whether isolated NMES is superior to swallowing therapy.
Nevertheless, in stroke patients with dysphagia, it is strongly recommended to define an individualized therapeutic plan, with early use of behavioral intervention, which should include rehabilitation and compensatory strategies such as swallowing exercises, environmental/postural changes, education on safe swallowing and modification of food consistencies 17), (20), (21), (22. As the instrumental assessment is the reference test for the assessment of swallowing, allowing the visualization of the physiology of swallowing and determining the pathophysiological and structural causes of dysphagia 14), (83, such information is essential for the design of an appropriate and effective therapeutic plan.
Despite the scant research carried out focusing on rehabilitation in the first 2 weeks after stroke 84), (85, early start of rehabilitation, 72 h after the onset of symptoms, has a greater impact on the recovery of swallowing function, as well as on the reduction of the incidence of respiratory complications 86. Despite not being entirely clear what the maximum neuroplasticity period is, it is particularly increased right after the stroke, during which the dynamic response of the brain to the injury is intensified and rehabilitation can be particularly effective 84. Therefore, a therapeutic intervention must be systematic, frequent and periodically reassessed 17), (20), (21.
Concerning rehabilitations strategies, none of the interventions has a significant impact on death or dependence 89. Acupuncture, behavioral intervention and RTMS may be effective, namely in reducing the proportion of patients with dysphagia, improving swallowing capacity and reducing penetration/aspiration 79), (81. However, the results may be due to chance, given the reversibility of the clinical condition. Furthermore, these results are supported by low-quality evidence. The AHA/ASA recommend that behavioral interventions and acupuncture may be considered in dysphagia treatment 22.
Analysis on the effectiveness of noninvasive neurostimulation techniques in patients with post-stroke dysphagia suggests benefits in the use of RTMS, TDCS and NMES, however with superior benefits for RTMS 80. Recommendations made in different international guidelines are contradictory concerning pharmacological treatment 21), (22, therefore preventing any recommendation. Caution is recommended in interpreting these results given the high heterogeneity of the studies, thus not allowing to make recommendations on the more effective protocols.
More recent evidence suggests a reduction in penetration/aspiration with behavioral intervention 87 and NMES 88. Improvement of swallowing functions was identified with behavioral intervention 87, NMES 88, RTMS 89 and acupuncture 90.
6.3 Certainty of Evidence of Effects
Most studies have high heterogeneity in intervention and outcome measurements. The quality of the evidence produced is moderate to low, mainly due to the lack of true randomization considering the difficulty of concealing the participant’s allocation and/or the intervention, which, in studies of this nature, proves to be a challenge. The apparent contradictions identified in the different clinical guidelines result from a set of factors that have highlighted the constraints of research in this area: the lack of uniformity in the assessment of dysphagia and the diversity of existing measurement instruments (whether clinical or instrumental); the lack of uniformity in measuring the results of interventions; the variability of available treatments and possible combinations, with no standardized interventions; the low methodological quality and high risk of bias in most published studies; the high probability of reversibility of the clinical condition (a significant percentage of patients spontaneously recover the ability to swallow), which means that the positive results of the studies may be due to chance 79), (80), (81. Therefore, the estimation of the true effect/efficacy of interventions is limited.
6.4 Summary of Delphi Panel’s Results
From the first Delphi round, the disagreement of the experts is worth noting regarding the uncertainty in the use of the NMES, which led to the elaboration of a specific recommendation. The first draft of recommendations was to advise against the use of NMES, due to the lack of high-quality evidence to support it.
6.5 Conclusions and Research Need for this Recommendation
Defining a therapeutic intervention for dysphagic stroke patients is, certainly, one of the major difficulties for health care providers. There is not sufficient evidence to determine whether improvement in swallowing function is due to the spontaneous recovery or due to the treatment, which enhances the need for systematic assessment and management of these patients. For research, constraints already identified must be addressed: the need for a clear definition of the time elapsed after the stroke at the time of recruitment, seeking to reach large samples, ideally from different centers; in the absence of proven intervention, participants in the control group should only receive standard care; the use of similar research methods thus allowing comparison, and the standardization of results and outcome measures. For outcome measurements and, additionally, functional outcomes (death and dependence), the proportion of patients who develop respiratory infection or pneumonia or show signs of aspiration and results relevant to the health economy such as length of stay, as well as the quality of life, should be assessed.
7 Clinical Question
Does modifying the consistency of food and liquids compared to standard feeding decrease mortality and morbidity or improve health outcomes in stroke patients?
7.1 Recommendation Statement(s)
The increase in the viscosity of liquids reduces the risk of aspiration; however, thickened liquids increase the risk of post-swallowing oral and pharyngeal residue. It is recommended that modification of food consistencies and the use of thickeners only be prescribed after clinical and/or instrumental evaluation. It is recommended to repeat assessments at regular intervals.
COR I
LOE C-EO
The use of thickeners decreases fluid intake. It is recommended to thicken fluids in patients with dysphagia for fluids. However, it is recommended to closely monitor fluid intake due to insufficient intake risk.
COR I
LOE C-EO
Even with changes in food consistencies and fluid viscosity, patients with dysphagia are at increased risk of malnutrition, dehydration and pneumonia. Therefore, it is recommended that they are closely monitored for these complications.
COR I
LOE C-EO
7.2 Summary of Evidence
The modification of food consistencies and thickening of liquids is probably the compensatory strategy mostly used in patients with dysphagia; however, the recommendation for its use is much more based on a consensus of good practice than on evidence from research 91), (92), (93), (94. Evidence on the safety and effectiveness of using strategies to increase the viscosity of liquids is scarce and, in the case of changes in food consistencies and use of mixed consistencies, it is residual 93.
Increasing the bolus viscosity increases the safety of swallowing, and the use of thickeners decreases penetration/aspiration; however, it increases the post-swallowing residue which can result in post-swallowing airway invasion 93. In addition, it has an impact on the physiology of swallowing with an increase in the tongue pressure pattern, but without impact on airway involvement and with controversial effect on oral and pharyngeal transit times, beginning of the opening of the upper esophageal sphincter and bolus speed 94. The use of thickened liquids results in an increased risk of dehydration 95, a higher prevalence of silent aspiration and worse quality of life 96. The use of thickeners may nullify the effect of drugs and the likelihood of error in the administration of drug therapy is much higher in dysphagic patients 97; however, there is insufficient evidence or consensus to formulate recommendations.
Patients report the experience of using thickeners as unpleasant, and this displeasure may negatively influence adherence to this therapeutic strategy, hydration status and quality of life 98. They also point out the lack of sensorial appeal as an important basis for the displeasure in relation to dietary changes and thickening of liquids, in addition to the fact that the involvement and understanding of the reasons for the prescription are low, leading to uncertainties about the treatment. It should be noted that it is recommended that, based on an individual assessment and decision and regular monitoring, additional access to nonthickened water be given to patients with liquid aspiration 21. In fact, the implementation of protocols for the intake of unthickened water under specific conditions may improve the quality of life 99; however, there are barriers to its implementation, namely the nurses’ lack of expertise in performing oral hygiene, lack of adherence to the rules of the protocols, perception of the increased workload for nurses and an established culture for the use of thickeners 99.
7.3 Certainty of Evidence of Effects
Despite the strong consensus amongst experts, nationally and internationally, it must be stated that there are limited data to support any of the recommendations. In fact, dietary modifications and fluid thickening are costly interventions without empirical support for their use, and there is growing evidence that they do more harm than good, for example, dehydration.
7.4 Summary of Delphi Panel’s Results
All recommendations achieved full agreement from all experts in the first round.
7.5 Conclusions and Research Need for this Recommendation
The use of dietary and fluid modifications in dysphagic patients depends on a thorough assessment of the patient’s ability to swallow, considering the increased risk of dehydration and post-swallowing residue. The main limitations on research in this field are the small sample size, including participants with multiple clinical conditions, heterogeneity in the methods and measurement of results, as well as the lack of uniformity in the definition and terminology of viscosity levels, which must be addressed in future research. It is also necessary to deepen the research regarding the administration of medication in dysphagic patients.
Conclusion
These recommendations aimed to gather the most recent evidence and highlight points of convergence in the existing recommendations for assessment and management of dysphagia in stroke patients. The various studies included in these recommendations make it possible to understand the impact that dysphagia has on a patient from a clinical point of view. Respiratory complications have been the main focus of research worldwide and the commitment to nutrition, hydration and quality of life of the person and family has not deserved the same attention. There is little evidence about the quality of life of stroke patients with dysphagia and their families.
These clinical recommendations are an overview of the most recent evidence translated into clinically relevant statements. In implementing recommendations at the local level, health professionals should identify facilitators and barriers to evidence-based practice within their own contexts and determine the best strategies to address local needs. Where change is needed, initial and continuing training on all recommendations is essential and relevant.
What this Work Adds to Current Practice
To our knowledge, these are the first specific national recommendations for the assessment and management of dysphagia in stroke patients and will provide health care practitioners with evidence-based guidelines translated into clinically relevant statements to assist in the decision-making process.
Limitations
Most of the evidence found is from moderate to low quality and denotes the need for further research to identify the best therapeutic options for these patients.
Patients were not involved in the elaboration of these recommendations, and this is acknowledged as one of its limitations. Due to the low quality of evidence, most recommendations are grounded in experts’ opinions. This might result in potential issues related to the diversity of clinical contexts and practices limiting their applicability. Recommendations should be reviewed within 5 years or if relevant evidence is identified before that period.
Statement of Ethics
This paper corresponds to a systematic literature review followed by a multidisciplinary panel of experts. All these experts were informed about the study aims, and participation was voluntary and anonymous. The consent was assumed after the participation of experts.
Funding Sources
No funds, nor any sort of payment, were received. CEISUC/CIBB is funded by national funds through FCT - Foundation for Science and Technology, I.P., under the Multiannual Financing of R&D Units 2020-2023.
Author Contributions
Conceptualization: Isabel J. Oliveira, Germano R. Couto, Pedro L. Ferreira. Data curation: Isabel J. Oliveira, Rosa V. Santos, Ana M. Campolargo, Cláudia Lima. Formal analysis: Isabel J. Oliveira, Rosa V. Santos, Ana M. Campolargo, Cláudia Lima. Investigation: Isabel J. Oliveira, Rosa V. Santos, Ana M. Campolargo, Cláudia Lima. Methodology: Isabel J. Oliveira, Germano R. Couto, Pedro L. Ferreira. Project administration: Isabel J. Oliveira, Germano R. Couto, Pedro L. Ferreira. Supervision: Germano R. Couto, Pedro L. Ferreira. Validation: Germano R. Couto, Pedro L. Ferreira. Writing - original draft: Isabel J. Oliveira, Germano R. Couto, Pedro L. Ferreira. Writing - review and editing: Isabel J. Oliveira, Germano R. Couto, Rosa V. Santos, Ana M. Campolargo, Cláudia Lima, Pedro L. Ferreira.